FORT BRAGG, NC—Immunization rates of human papilloma virus (HPV) have risen among active duty forces, but many fail to complete the full vaccine series, despite recommendations from the Defense Health Agency.
“DHA fully endorses CDC guidance regarding the HPV vaccine and encourages service members who have not received the full HPV vaccine series to initiate or complete their immunization,” said Bruce McClenathan, MD, medical director of the DHA’s South Atlantic Regional Vaccine Safety Hub, Immunization Healthcare Branch, at Fort Bragg, NC.
While the HPV vaccine is not mandatory for U.S. military servicemembers, each service has implemented policies that encourage members and beneficiaries to receive the vaccine.
The vaccine has the potential to eliminate or dramatically reduce the incidence of several cancers. “HPV is responsible for nearly all types of cervical cancer and is a contributing factor for many other cancers in both men and women,” noted McClenathan.
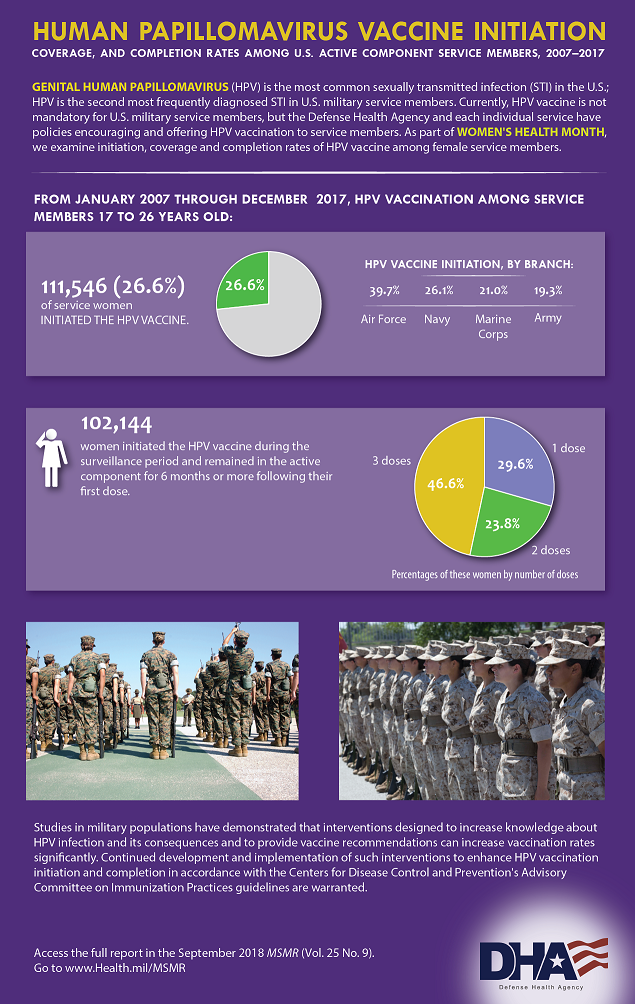
Between January 2007 and December 2017, more than 233,000 servicemembers between the ages of 17 and 26 received the HPV vaccine. Among women, the number was 11,546, or 26.6% of those eligible. A much lower percentage of men initiated the series. The 121,657 men who received any HPV vaccine represented just 5.8% of those eligible, according to a recent study in Military Surveillance Monthly Report (MSMR).1
Among active-duty forces, 46.6% of eligible women who began the vaccine series received the recommended three shots during the study period. That’s a slight increase from the 45.5% completion rate found in a study of women in the U.S. Armed Forces in the five years following the U.S. Food and Drug Administration approval of the first HPV vaccine in 2006. 2
Men who began the HPV series had completion rate of 35.5% between 2007 and 2017.
The rates of vaccination initiation varied substantially among the services. The Air Force had much higher rates of HPV vaccination over the 10 years studied in the recent report, with 39.7% of eligible women and 16.5% of eligible men receiving at least one dose of the vaccine. The Navy had the second highest rates, at 26.1% for women and 4.2% of men. In the Marine Corps, the rates were 21% of women and 2.1% of men; 19.3% of female soldiers and 3.6% of male soldiers received the vaccine.
The national Centers for Disease Control and Prevention notes that the virus is extremely common and that “most people will become infected at some point in their life.” While many of those infections will resolve on their own, thousands of cases of cancer are attributed to the virus each year.
Universal HPV vaccination could eradicate cervical cancer, which is often diagnosed after it has already metastasized. Cervical cancer has a five-year survival rate of 69% among white women and 56% among black women, according to the American Society of Clinical Oncology. More 12,000 women in U.S. are diagnosed with the disease each year and 4,000 die of it.
The vaccine protects men against cancer, too. HPV causes 71% of penile cancers as well as 90% of all anal cancers and 72% of throat or oropharyngeal cancers, according to public health officials. Men are twice as likely to develop oropharyngeal cancer than women, and research from the Mayo Clinic suggests that about 23,000 cases will be diagnosed this year.
The vaccine also protects against genital warts and respiratory papillomatosis in men and women.
Since the introduction of the vaccine, the incidence rates of genital HPV infections among servicemembers has dropped sharply. From 2007 to 2016, the infection rate declined 75% among all active duty women, according to a report in MSMR.3 The overall reduction was driven by a more than 90% drop in women under age 24, who “may have been vaccine for HPV prior to entering service,” the authors said.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) recommends three doses of the HPV vaccine for patients ages 15 to 26 with the second dose given two months after the first and the third dose given four months later. Patients aged 9 to 14 should receive just two doses six months apart.
The CDC recommends giving the vaccine to boys and girls at age 11 or 12, but “catch up” vaccinations may be administered until much later.
This fall, the Food and Drug Administration raised the approved age for Gardasil 9 to include men and women up to age 45. Gardasil 9, which covers nine strains of HPV and is the only vaccine now recommended and distributed in the U.S.
The “approval represents an important opportunity to help prevent HPV-related diseases and cancers in a broader age range,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research. “The Centers for Disease Control and Prevention has stated that HPV vaccination prior to becoming infected with the HPV types covered by the vaccine has the potential to prevent more than 90% percent of these cancers, or 31,200 cases every year, from ever developing.”
Clark LL, Stahlman S, Taubman SB. Human papillomavirus vaccine initiation, coverage, and completion rates among U.S. active component service members, 2007-2017. MSMR. 2018 Sep;25(9):9-14.
Maktabi H, Ludwig SL, Eick-Cost A, Yerubandi UD, Gaydos JC. Quadrivalent human papillomavirus vaccine initiation, coverage, and compliance among U.S. active component service women, 2006-2011. MSMR. 2012 May;19(5):16.
Stahlman S, Oetting AA. Sexually transmitted infections, active component,U.S. Armed Forces, 2007-2016. MSMR. 2017 Sep;24(9):15-22.