PITTSBURGH—In more good news for veterans who have received treatment for hepatitis C virus, VA researchers have found that treatment not only reduces the risk of complications from liver disease, it also dramatically reduces the risk of cardiovascular disease.
VA researchers led by Adeel Ajwad Butt, MD, MS, a research associate with the Pittsburgh VAMC and professor of Medicine and Healthcare Policy and Research at Weill Cornell Medical College in New York City, presented results of their study at IDWeek 2018. The study also appeared in Gastroenterology.1
The VA’s aggressive treatment of HCV in veterans since the advent of direct-acting antivirals has changed the long-term view for many veterans, making management of other chronic conditions even more important, according to the study authors.
“With cure rates of >90% with DAAs and a clear survival benefit with treatment, more HCV-infected persons will live longer. Reducing CVD risk will be increasingly important in these patients,” they wrote.
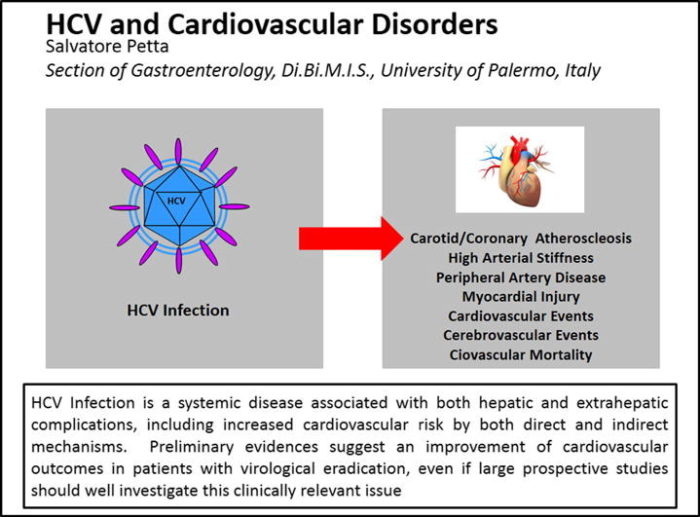
Source: J Adv Res. 2017
Mar;8(2):161-168. doi: 10.1016/j.jare.2016.06.001. Epub 2016 Jun 18.
The team found that veterans treated with DAAs, the current standard of care, were about half as likely to experience an incident cardiovascular event as veterans who had not received any treatment. Patients who were treated with earlier therapies also saw a reduction in risk, but not as much.
“Our study provides strong evidence that the benefits of treating HCV go beyond the liver, which includes reducing the risk of CVD events,” Butt said.
Other studies have come to conflicting conclusions as to whether HCV treatment reduced the risk of developing cardiovascular disease, the authors noted. The size of the VA study gives this research added weight as does its comparison of treatments.
“Our study adds significant new information by assessing older pegylated interferon/ribavirin and newer DAA regimens and their effect on CVD outcomes,” the authors noted.
Butt and his colleagues identified 242,689 patients with chronic HCV in the Electronically Retrieved Cohort of HCV-Infected Veterans cohort. They excluded patients with hepatitis B virus, human immunodeficiency virus or known cardiovascular disease and those without sufficient recorded data to determine whether they achieved sustained virologic response after treatment. Veterans who had received more than one treatment regimen were also excluded.
The final analysis included 4,436 veterans (26%) who had been treated with a pegylated interferon and ribavirin regimen and 12,667 veterans (74%) who had received direct-acting antivirals matched by age, race, sex and baseline values to an equal number of untreated controls. Nearly all the participants (96%) were male, 56% were white, and 27% were black. The median age was 58 years.
Patients who were untreated and those who received older regimens with pegylated interferon and ribavirin were followed for 10 years. “Follow up was shorter for those treated with direct-acting antiviral regimens, since the newer DAA regimens were approved more recently, and most veterans started treatment with those regimens in 2015 or later,” Butt told U.S. Medicine.
The researchers defined a cardiovascular disease event as the presence of at least one inpatient or at least two outpatient codes for acute myocardial infarction, unstable angina, congestive heart failure, peripheral vascular disease, percutaneous transluminal coronary angioplasty, coronary artery bypass grafting or stroke, Butt said. The researchers looked for CVD events that occurred at least 12 weeks after the start of treatment for hepatitis C.
Twice as Frequently
Incident CVD events occurred almost twice as frequently in the untreated group (13.8%) than in the treated group (7.2%). The incidence rates were 30.9 per 1,000 patient-years of follow-up in the control group and 20.3 per 1,000 patient-years in the treated group.
Two factors contributed to reduced risk of an incident cardiovascular event among treated patients: attaining sustained virologic response and use of direct-acting antivirals, the researchers found. Patients who achieved SVR had a 13% reduction in risk when compared to treated patients who did not achieve SVR.
The difference in risk was most striking between the veterans treated with DAAs and those that were not treated. Patients who received DAAs had a 43% reduction in risk compared to untreated patients, while those who received the pegylated interferon and ribavirin combination saw just half of that risk reduction, 22%.
The authors concluded that “”HCV treatment is associated with a significantly reduced risk of cardiovascular events. Those treated with a DAA regimen and those who achieve SVR have a greater benefit.”
Butt noted that the benefit of the new treatments extended even further. Not only does treatment with DAA reduce the risk of an incident cardiovascular event in patients who had been positive for HCV, the newer drugs can also be used in patients who already have cardiovascular disease.
“In the interferon-era, pre-existing CVD was a relative contraindication to treatment due to the risk of ribavirin-induced anemia which may have precipitated a CVD event. However, in the DAA era, presence of CVD should not be a contraindication to treatment,” he said.
Still, Butt advised that “patients with HCV should be actively screened for CVD risk factors and receive interventions to reduce such risk,” including smoking cessation counseling and lipid-lowering therapy.
Patients with advanced fibrosis had a higher risk of CVD events compared to those with minimal or no fibrosis, but HCV treatment reduced the risk across all levels of fibrosis.
The authors noted that “taken together, these observations strengthen the mounting evidence of the association between advanced liver fibrosis and CVD events, and a significant reduction in such risk with treatment.”
Butt AA, Yan P, Shuaib A, Abou-Samra AB, Shaikh OS, Freiberg MS. Direct-Acting Antiviral Therapy for HCV Infection Is Associated With a Reduced Risk of Cardiovascular Disease Events. Gastroenterology. 2018 Nov 13. pii:S0016-5085(18)35264-8.
