WASHINGTON — While the availability of novel therapies is making the future brighter for non-Hodgkin’s lymphoma (NHL) patients, new treatments also are coming on line for Hodgkin’s lymphoma, which is a hematological cancer distinct from NHL.
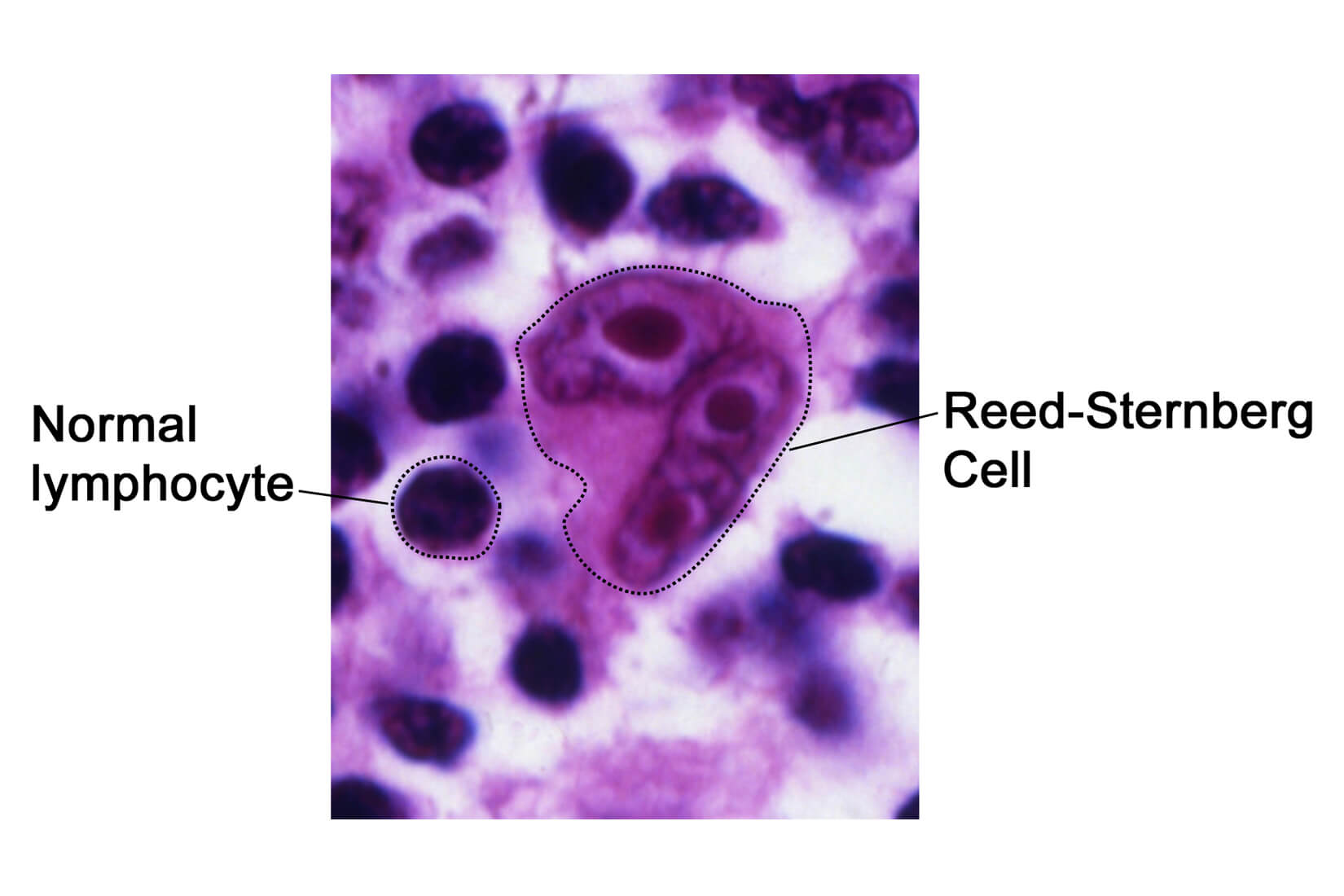
While both Hodgkin’s and non-Hodgkin’s lymphoma are lymphocyte malignancies, Hodgkin lymphoma is distinguished by the presence of Reed-Sternberg cells, which are mature B cells that have become malignant, are unusually large, and carry more than one nucleus. The symptom leading to diagnosis usually is enlarged lymph nodes.
Both cancers are recognized by the VA as presumptive diseases associated with exposure to Agent Orange or other herbicides during military service. Because of that exposure, veterans and their survivors might be eligible for VA benefits related to those diseases. Hodgkin’s, however, has a much lower rate among veterans, 0.25% of all cancers diagnosed at the VA, compared to NHL at 3.34%, according to a report last year in Military Medicine.1
Based on approval in March by the Food and Drug Administration, brentuximab vedotin also now can be used to treat adult patients with previously untreated stage III or IV classical Hodgkin lymphoma (cHL) in combination with chemotherapy.
“Today’s approval represents an improvement in the initial treatment regimens of advanced Hodgkin lymphoma that were introduced into clinical practice more than 40 years ago,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research. “This approval demonstrates our commitment to approving advancements in treatment that give prescribers and patients different options for care.”
The drug, marketed as Adcetris, combines an antibody and drug, allowing the antibody to direct the drug to a target on CD30 lymphoma cells. Adcetris has also been previously approved by the FDA to treat cHL after relapse or cHL after stem cell transplant when a patient is at a high risk of relapse or progression.
In terms of NHL, Adcetris is approved to treat, systemic anaplastic large cell lymphoma (ALCL) after failure of other treatment, and primary cutaneous ALCL after failure of other treatment.
The latest FDA approval, for adult patients with previously untreated stage III or IV cHL was based on a clinical trial comparing Adcetris plus chemotherapy — Adriamycin [doxorubicin], vinblastine and dacarbazine (AVD) — to a chemotherapy-only regimen common for cHL treatment — AVD plus bleomycin (ABVD).
After the 1,334 participants received an average of six 28-day cycles of treatment, those treated with Adcetris plus AVD were 23% less likely to experience progression, death, or initiation of new therapy compared with those receiving ABVD. Overall, 18% of patients in the Adcetris plus AVD group experienced disease progression, death, or began new therapy vs. 22% of those on ABVD.
The FDA granted the application to Seattle Genetics, Inc. with its priority review and breakthrough review designations.
11. Zullig LL, Sims KJ, McNeil R, Williams CD, Jackson GL, Provenzale D, Kelley MJ. Cancer Incidence Among Patients of the U.S. Veterans Affairs Health Care System: 2010 Update. Mil Med. 2017 Jul;182(7):e1883-e1891. doi: 10.7205/MILMED-D-16-00371. PubMed PMID: 28810986; PubMed Central PMCID: PMC5650119.
