Research from the University of Michigan, Johns Hopkins and other medical centers has been raising concerns about the accuracy of pulse oximeters in certain racial groups almost since the COVID-19 pandemic began.
Slow Postal Service Hits Veterans Hard With Medication Delivery Delays
The U.S. Postal Service (USPS) has long been the most popular government institution in the country, but cascading delays in the last eight months have threatened its preferred position as both businesses and individuals suffer from lost and stranded mail and packages.
FDA Updates Covid-19 Vaccine Label to Urge Use of ‘Sixth Dose’
The Food and Drug Administration has approved an updated label for the Pfizer-BioNTech vaccine, urging that the sixth dose in vaccine vials be used.
Study: Use Fluoroquinolones Only for Serious Infections Without Alternatives
Prescriptions for fluoroquinolones have been dropping over the past decade at the VHA and elsewhere. A new study pointed out that reasons include both a greater emphasis on antimicrobial stewardship, as well as growing provider awareness of serious adverse drug reactions.
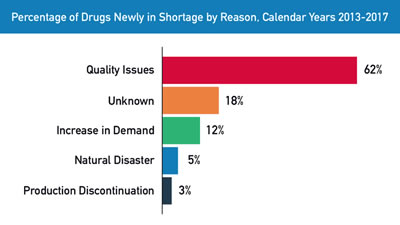
Drug Shortages Put Veterans at Risk, Often for Months at a Time
VA Is Part of Group Looking at National Solutions WASHINGTON—Drug shortages have plagued hospitals with their frequency, breadth and duration over the past decade, with more than 100 drugs in shortage as of mid-November, according to the U.S. Food and Drug...
VA Study Addresses Concerns About Anti-Epileptic Drugs, Suicidal Behavior Link
In 2008, the U.S. Food and Drug Administration issued an alert about increased risk for suicidal ideation and behavior for patients taking anti-epileptic drugs (AEDs).