CHICAGO—No healthcare system has been more affected by the dramatic rise in hepatocellular carcinoma than the VA.
In 2000, the VA had 1,361 patients diagnosed with the disease. By 2005, the number of veterans diagnosed with HCC had more than tripled to 4,989, according to the VA. By 2015, the number had doubled again, reaching 11,250. The number of veterans with HCC receiving care declined slightly to 9,905 in 2018 but is expected to resume its upward trend.
One of the reasons for that is a disturbing trend in the military: Over the last 17 years, the incidence of nonalcoholic fatty liver disease among active-duty servicemembers leapt from 12.6 cases per 100,000 person-years to 152.8.
That’s why development of new treatment options for HCC is so critical for VA clinicians and their veteran patients.
Earlier this year, the Food and Drug Administration approved cabozantinib for patients with HCC who have been previously treated with sorafenib.
Now, the Phase 3 (COSMIC-312) study is looking at cabozantinib in combination with atezolizumab vs. sorafenib (S) in patients with advanced HCC who have not received previous systemic anticancer therapy.
A description of the study, led by University of California San Francisco researchers, was presented in a poster at the recent 2019 American Society for Clinical Oncology Annual Meeting in Chicago.1
The study team pointed out that cabozantinib inhibits tyrosine kinases involved in tumor growth, angiogenesis and immune regulation, including MET, VEGFR and TAM kinases (Tyro3, AXL, MER).
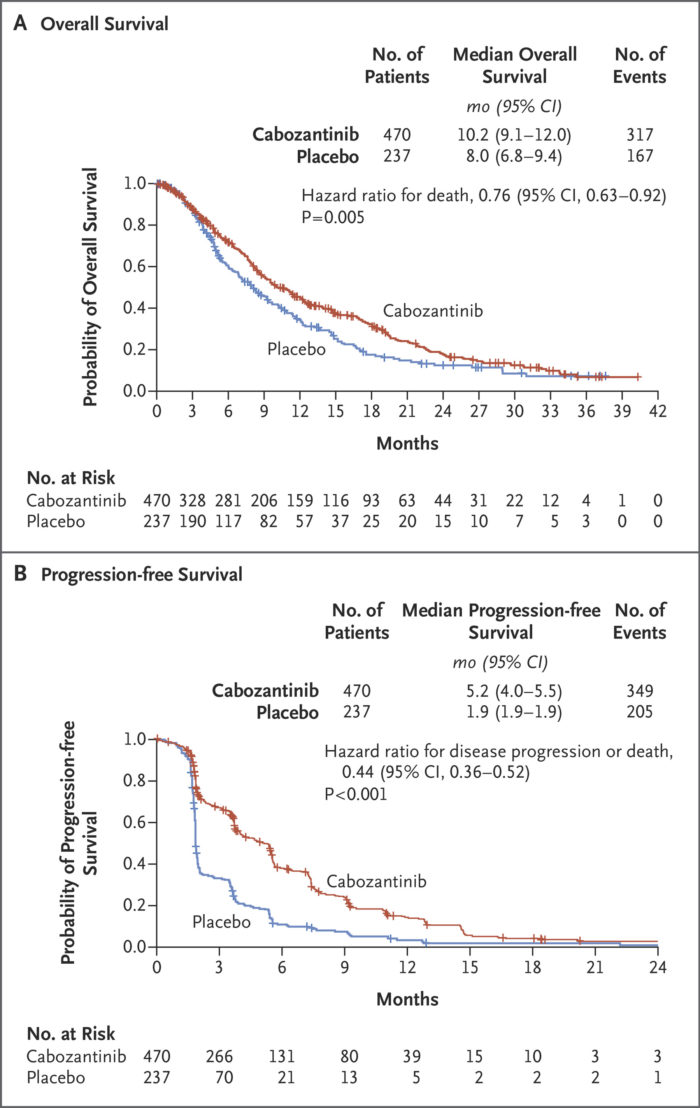
Cabozantinib currently is approved for treatment of advanced HCC after prior sorafenib therapy based on improved overall survival vs. placebo in the Phase 3 CELESTIAL trial.2
Standard of care for first-line treatment of advanced HCC is tyrosine kinase inhibition with sorafenib or lenvatinib, and Phase 3 trials of immune checkpoint inhibitors in first- and second- line advanced HCC are ongoing, according to the current researchers.
They suggested that cabozantinib might promote an immune-permissive tumor environment, which could enhance response to ICIs and is being evaluated in combination with the anti-PD-L1 antibody atezolizumab in multiple tumor types, including HCC, in a Phase 1 study. Preliminary clinical activity and safety have been established in advanced renal cell carcinoma, the report noted.
Atezolizumab, in combination with bevacizumab, an anti-VEGF antibody, has shown preliminary clinical activity in first-line advanced HCC, the study team added. Researchers presented the study design of a Phase 3 trial of cabozantinib in combination with atezolizumab vs. sorafenib in patients with advanced HCC as first line treatment.
Eligibility criteria for the international, randomized, open-label Phase 3 trial (NCT03755791) includes age over 18, BCLC Stage B or C, Child-Pugh A, ECOG PS 0 or 1, and measurable disease per RECIST 1.1.
Patients are to be randomized 6:3:1 to an experimental arm of cabozantinib (40 mg qd) plus atezolizumab (1200 mg infusion q3w), a control arm of sorafenib (400 mg bid) and an exploratory arm of cabozantinib monotherapy (60 mg qd). Researchers said that 640 participants are planned at about 200 sites globally.
Randomization is stratified by:
- Disease etiology (HBV [with or without hepatitis C], HCV [without hepatitis B], or other);
- Region (Asia, other); and
- Presence of extrahepatic disease and/or macrovascular invasion (yes, no).
Defined as coprimary endpoints are overall survival and progression-free survival, with the objective response rate being a secondary endpoint.
Study authors reported that additional endpoints include safety, pharmacokinetics and correlation of biomarker analyses with clinical outcomes.
1 Kelley RK, Cheng A, Braiteh FA, Park J, et. Al. Phase 3 (COSMIC-312) study of cabozantinib (C) in combination with atezolizumab (A) versus sorafenib (S) in patients (pts) with advanced hepatocellular carcinoma (aHCC) who have not received previous systemic anticancer therapy. J Clin Oncol 37, 2019 (suppl; abstr TPS4157)
2 Abou-Alfa G, Meyer T, Cheng A, El-Khoueiry AM, et. Al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med 2018; 379:54-63 DOI: 10.1056/NEJMoa1717002
