VA Research Seeks to Determine Who Is at Highest HCC Risk
More than 100,000 VA patients have been cured of hepatitis C with direct-acting antiviral treatment. Even though HCV is the leading cause of hepatocellular carcinoma in the United States, curing the infection only reduces the risk; it doesn’t entirely eliminate it. That’s why the VA has continued with research to determine who is at greatest risk of HCC and is looking at expanding screening.
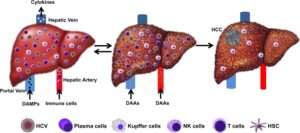
Hepatic adaptive response to inflammatory stress progresses to cirrhosis. Blood supply to the liver by both the hepatic artery and the portal vein brings potential pathogens, including HCV. Kupffer cells and sinusoidal endothelial cells recognize HCV-derived pathogen-associated molecular pattern (PAMP). The activation of the innate immune response through Kupffer cells recruit new adaptive immune cells (CD4 and CD8 T cells) and B cells. Sustained inflammation can lead to liver fibrosis, cirrhosis, and HCC. Chronic hepatitis is a physiological adaptation that can be reversible after HCV cure. Pathological adaptation of the inflamed liver can lead to cirrhosis and HCC. Source:
J Hepatocell Carcinoma. 2020; 7: 45–76. Published online 2020 Apr 15. doi: 10.2147/JHC.S221187
HOUSTON — In the last six years, the VA has cured chronic hepatitis C (HCV) infections in more than 100,000 veterans using direct-acting antivirals. As HCV has been the leading cause of hepatocellular carcinoma (HCC) in the U.S. and the VA, curing the infection reduces the risk of developing liver cancer.
Unfortunately, the risk is not entirely eliminated.
Of the individuals who have chronic hepatitis C infection, 15% to 20% will go on to develop cirrhosis of the liver. Patients with cirrhosis have 1% to 5% annual risk of developing HCC, according to the national Centers for Disease Control and Prevention. That risk doesn’t go away immediately for patients with cirrhosis who achieve sustained virologic response (SVR) or cure.
“The risk [of HCC] remains high in patients who have progressed to cirrhosis already,” said Fasiha Kanwal, MD, MSHS, an investigator in the Clinical Epidemiology & Comparative Effectiveness Program at the Center for Innovations in Quality, Effectiveness and Safety at the Michael E. DeBakey VAMC, chief of the Gastroenterology and Hepatology Section at the Baylor College of Medicine, both in Houston, and editor-in-chief of Clinical Gastroenterology and Hepatology when she spoke with U.S. Medicine last year.
Thirty percent to 40% of veterans had already developed cirrhosis when they received treatment for HCV.
With more than 30,000 veterans at elevated risk of HCC, the VA has led national research into the relationship between HCV infection and treatment and development of liver cancer.
Cirrhosis and FIB-4
“[A]mong patients with established cirrhosis before antiviral treatment, a substantial absolute risk of HCC persists after SVR, even though it is significantly reduced compared to untreated patients and those who did not achieve SVR,” according to study by George N. Ioannou, MD, MS, a gastroenterologist at the Veterans Affairs Puget Sound Healthcare System and professor of Gastroenterology at the University of Washington, both in Seattle, and colleagues.1
The risk is not same for all veterans with cirrhosis or, as the researchers discovered, for all veterans without cirrhosis at the time of treatment.
The team analyzed outcomes for 48,135 veterans who achieved SVR between 2000 and 2015. Of those, 9,784 had cirrhosis prior to treatment for HCV. Direct-acting antiviral (DAA) only regimens were used in 29,033 patients, while 19,102 received interferon-based therapies.
A total of 1,509 patients developed HCC more than 180 days after treatment initiation. Patients who developed HCC within 180 days of treatment initiation were excluded from the study.
The researchers found that the risk of HCC was significantly associated with cirrhosis and Fibrosis-4 (FIB-4) liver fibrosis score prior to treatment. Veterans with pretreatment cirrhosis and FIB-4 of 3.25 or higher before and after achieving SVR on direct-acting antiviral therapies had an annual incidence of HCC of about 5%. Those that had FIB-4 scores below 3.25 before and after treatment had a much lower annual incidence, less than 1%.
Not all veterans who had not developed cirrhosis were in the clear, however. Among veterans without cirrhosis at the time of treatment, those who had FIB-4 scores of 3.25 or higher had an absolute HCC risk of more than 2% per year. Notably, about 80% of patients without cirrhosis who had scores of 3.25 prior to treatment experienced a drop in FIB-4 within a year of achieving SVR, which reduced their risk below 1% annually.
The researchers found that among DAA-treated patients, the annual incidence of HCC declined over time. Those who had FIB-4 scores of 3.25 or greater prior to treatment saw a drop from 3.8% in year 1 to 2.4% in year 4. Veterans with FIB-4 scores below 3.25 at initiation experienced a decline in annual incidence from 1.4% in year 1 to 0.5% in year 4.
In both groups, the decline was statistically significant, but the researchers cautioned that they were only able to evaluate patients for four years because of the relative newness of the therapy. In interferon-treated veterans, the researchers identified no discernable decline in HCC risk over time with 10 years of data available.
Because of the high risk of HCC, the VA recommends regular screening for all veterans with cirrhosis. Ioannou and his colleagues recommended expanding surveillance to include veterans who had FIB-4 of 3.25 or higher, even without cirrhosis.
In light of HCC risk and increased screening, VA is expanding its formulary, including the recent addition of lenvatinib, marketed as Lenvima, which is the latest drug approved for first-line treatment of the cancer.
