MCL Is a Presumptive Condition for Veterans Exposed to Agent Orange
Most patients with mantle cell lymphoma, which is considered a presumptive condition for veterans exposed to Agent Orange, face a rapidly progressive disease and higher mortality rates. New therapies are giving VA clinicians more options to help MCL patients. For example, the availability now of three Bruton tyrosine kinase [BTK] inhibitors is allowing treatment to be better matched to the needs of the patient.
In November 2019, after this study was published, the Food and Drug Administration granted accelerated approval to zanubrutinib (BRUKINSA, BeiGene, Ltd.) for adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy.
SYRACUSE, NY — The last few years have seen a dramatic shift in treatment for mantle cell lymphoma as multiple new drugs gained approval.
Mantle cell lymphoma (MCL) accounts for between 2% and 6% of all non-Hodgkin lymphomas (NHLs). Until recently, MCL was simply considered a subtype of chronic lymphocytic leukemia with particularly poor prognosis. Increased understanding of the disease’s molecular biology has enabled development of new drugs that target specific pathways in MCL, offering better outcomes with lower rates of toxicity.
That’s particularly good new for veterans. As a non-Hodgkin lymphoma, MCL is considered a presumptive condition for veterans exposed to Agent Orange. The older age and predominantly male demographic of U.S. veterans also put them at greater risk for MCL. About three-quarters of MCL patients are male and the average age of diagnosis is 68 years.
While some patients develop an indolent form of MCL, most face a rapidly progressive disease with shorter durations of response to therapies as well as shorter periods of progression-free survival and overall survival compared to other lymphomas. Low-risk patients have a five-year survival rate of 60%.
In contrast, intermediate risk patients have a median overall survival of 51 months and those with high-risk disease have overall survival of just 29 months, noted Jeffrey J. Pu, MD, PhD, associate professor of medicine, pathology, and pharmacology, Upstate Cancer Center, State University of New York Upstate Medical University, and Syracuse VA Hospital, both in Syracuse, NY, in a review of the disease.1
“When people think about mantle cell lymphoma, they usually think about the aggressive subtype,” according to Michael Kolodziej, MD, vice president and chief innovation officer at ADVI Health in Washington, DC, in a discussion of new National Cooperative Cancer Network (NCCN) guidelines for the disease. “And the aggressive subtype of mantle cell lymphoma is treated much like, interestingly, aggressive lymphomas like diffuse large B-cell lymphoma.”2
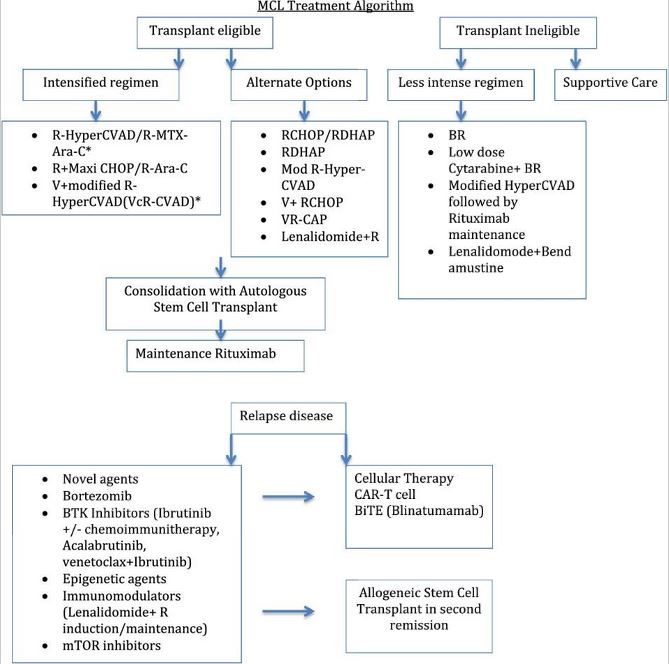
For patients who are transplant eligible, the goal is to have them achieve remission with intensive induction and consolidation therapies and then proceed to autologous stem cell transplant (autoSCT) followed by maintenance therapy with rituximab. For those who are not transplant eligible, the hope is to knock the disease back as long as possible. Bendamustine plus rituximab has been the most common therapy for these patients as clinical trials demonstrated longer progression-free survival compared to the two other most common combinations in elderly patients.
Both groups face a high rate of relapse, however, as there is no curative treatment for MCL.
BTKs Change Treatment
“Until recently, treatment of relapsed and refractory mantle cell lymphoma was really not particularly good. But that changed really, I think, when it became clear that this new class of drugs, the Bruton tyrosine kinase [BTK] inhibitors, were active in this disease,” Kolodziej said.
The first BTK for mantle cell lymphoma, ibrutinib, received U.S. Food and Drug approval for relapsed or refractory MCL in 2013. Acalabrutinib was approved in 2017 and zanubrutinib received accelerated approval from the FDA in November 2019.
“These drugs are highly effective in treating relapsed and refractory mantle cell lymphoma, often for a very durable period, often with quite acceptable toxicity,” Kolodziej said.
A team led by researchers at the VA Puget Sound Healthcare System found that 10 of 37 patients with MCL who underwent autoSCT between 2009 and 2017 at the Seattle VAMC experienced progression. Those who relapsed had median progression free survival of 1.8 years and median overall survival following progression of just 0.7 years.3
Notably, the three patients who survived for more one year all received the BTK ibrutinib.
While patients who relapse may receive chemotherapy again, “the NCCN guidelines recommend the efficacy of these agents and offers them as a treatment of choice,” Kolodziel said. “We’re seeing the BTK inhibitors becoming the predominant therapy.”
With three BTKs now available, Pu told U.S. Medicine that the “first choice depends on both the patient’s history and comorbidities, and current experience with that drug.” Because ibrutinib received approval first, most physicians have more experience with it, it is often used initially, he said, adding,. “However, acalabrutinib has a better [adverse events] profile, and oncologists may use it before ibrutinib if the patient has a bleeding tendency.”
As a second generation BTK, zanubrutinib “is thought to be more selective in terms of not hitting as many off-target kinases as some of the other drugs in its class,” said Ian Flinn, MD, of the Sarah Cannon Research Institute in Nashville, TN in a discussion on OncLive. “The hope is that by being more selective, you’re not going to have some of the off-target toxicities such as bleeding, cardiac effects, diarrhea, rash, and other potential problems.”4
Other New Therapies
Other new therapies are also being studied in MCL. “At research and early trial level, there are some successes with mammalian target of rapamycin (mTOR) inhibitors, but we still have problems finding a strong mTOR for hematological malignancy,” Pu said.
Chimeric antigen receptor-engineered T-cells (CAR T) and bi-specific T-cell engager therapy (BiTE) as well as allogeneic hematopoietic stem cell transplantation (alloSCT) are emerging as options for relapsed disease, often following BTKs or other newer therapies such as epigenetic agents, immunomodulators, mTORs, and bortezomib.
“CAR T is a way to go for relapsed/refractory MCL but the cost is too high,” Pu noted. “If putting financial barriers aside, the patient’s own physical condition and possible donor for alloSCT are the first factors to consider. Physically fit patients with a proper donor always go to transplant.”
“BiTE is very promising. I expect it will phase out CAR T in the near future,” he added. “It takes time to transfer science to practice. CAR T is just staging now; BiTE is actively following.”
- Ladha A, Zhao J, Epner, EM, Pu JJ. Mantle cell lymphoma and its management: where are we now? Exp Hematol Oncol. 2019;8(1):2. doi.org:10.1186/s40164.019.0126.0
- The Changing Treatment Landscape of B Cell Malignancies: MCL and CLL: NCCN Guidelines and Treatment Options. AJMC.com. Jan 21, 2020.
- Wu A, Graf SA, Burwick N, et al. Mantle cell lymphoma relapsed after autologous stem cell transplantation: a single-center experience. Blood Res. 2020;55(1):57–61. doi:10.5045/br.2020.55.1.57
- The Changing Landscape in Mantle Cell Lymphoma: Recent Approval of Zanubrutinib for R/R MCL. OncLive.com. February 20, 2020.
