MEMPHIS, TN—Because biologic therapies prescribed for inflammatory diseases increase the risk of activation of latent tuberculosis infections, some VAMCs recommend baseline testing of veterans starting these agents and annual testing thereafter. Several recent studies suggest that much of that testing could be unnecessary and that a more focused approach would be a better and more cost-effective strategy.
More than 100,000 veterans have chronic inflammatory conditions, such as rheumatoid arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis and psoriasis, all of which are commonly treated with biologic therapies.1,2
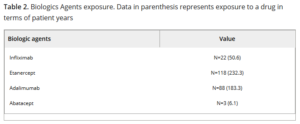
Biologic therapies for inflammatory diseases include tumor necrosis factor (TNF)-alpha inhibitors, integrin inhibitors, and certain other monoclonal antibodies such as abatacept, adalimumab, certolizumab pegol, etanercept, golimumab, infliximab, natalizumab, rituximab, tocilizumab, ustekinumab and vedolizumab.
Biologic agents target specific immune cells and disrupt the inflammatory cascade, reducing flare-ups and damage associated with these diseases. By disrupting the immune system, however, they also increase the risk of infection and, in the case of tuberculosis, the risk of reactivation of a latent infection.
Initial Screening
About 10% of people exposed to the organism that causes tuberculosis, Mycobacterium tuberculosis, go on to develop the disease. In others, the bacilli remain in the body at a low level but do not cause active disease. A change in immunological status can allow the bacilli to reproduce, even decades after first exposure. Reactivations of latent infections account for about 80% of all tuberculosis infections in the U.S.
“All patients should be screened (regardless of risk factors and prevalence area) prior to starting biologics,” suggested Debendra Pattanaik, MD, associate professor of medicine-rheumatology at the University of Tennessee Health Science Center in Memphis, TN, and first author of a study of TB screening among veterans receiving biologic therapy for rheumatic disease.3
“Patients that have risk factors should be screened with a dual testing strategy,” that includes repeat interferon gamma release assays (IGRA) if the first result is negative, he told U.S. Medicine. Conversely, “if there is low suspicion for infection, then you can retest [after a positive result] to ensure that it is not a false positive.”
Dual testing overcomes the shortcomings of both common tests for latent tuberculosis infections, IGRA and the tuberculin skin test. “False positives are more likely with TST since IGRAs are not affected by most non-tuberculous mycobacteria and bacillus Calmette-Guerin (BCG) vaccination,” Pattanaik noted. Further, IGRA tests such as the QuantiFERON‐TB Gold In‐Tube (QFT‐GIT) assay and T-SPOT.TB “may be more sensitive in immunosuppressed patients.”
Repeat Testing
But should all patients be tested every year after they start on biologics? Research by Pattanaik and others indicated that some patients should be screened annually, but most should not.
“Patients at risk of ongoing TB exposure should be rescreened annually,” Pattanaik said.
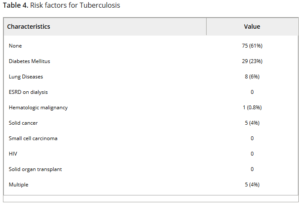
Exposure factors include living or working in prisons, homeless shelters and healthcare settings where tuberculosis is treated; close contact with someone who has TB or work in a microbiology lab that handles TB; and birth or extended residence in an area of high TB prevalence. Other factors that increase activation of latent tuberculosis include infection with human immunodeficiency virus, end-stage renal disease on hemodialysis, certain cancers and treatments, steroid therapy, diabetes, tobacco use, abnormal chest X-ray indicative of@healed tuberculosis and being malnourished or severely underweight.
“For patients who have screened negative or who have successfully completed LTBI therapy and who lack the potential for subsequent TB exposure, rescreening is probably unnecessary.”
Pattanaik and colleagues at the University of Tennessee identified 298 veterans who received biologic agents for rheumatic diseases at the rheumatology clinic of the Memphis VAMC between January 2003 and December 2014. Of those, 123 had long-term follow-up and multiple TB test results and were included in their study.
They found that only one person, 0.8% of the study population, seroconverted during the study period. That veteran had no known exposure to active TB or other risk factors, a previous negative test after the initial one, a negative chest X-ray and did not develop active tuberculosis. He received nine months of isoniazid therapy.
The annual incidence rate of seroconversion after a negative test was 2.5 per 10,000 patient years.
“The extremely low rate of seroconversion in our patients is likely due to the low prevalence rate of TB in the area served by this VA medical center,” the researchers wrote.
In areas with low rates of tuberculosis, apparent seroconversion may be suspect. The authors noted that previous studies have shown that repeat testing six months following a positive test returned negative results in the majority of cases. Similarly, results indicating seroconversion among healthcare workers in areas with low TB rates were typically false positives, with rates as high as 30 false positives for every true infection detected. Another study they cited found that between 50 and 80 patients immunocompromised patients with positive test results would need to be treated to prevent one case of TB.
While development of active TB can be catastrophic, treatment with isoniazid (isonicotinic acid hydrazide or INH) poses its own risks. The drug can cause seizures, liver injury, hepatitis and, occasionally, severe or fatal liver damage.
Based on the results, Pattanaik said, “we feel that risk of seroconversion for tuberculosis, even among veterans who have a lot of comorbidities, is extremely low and there is no need for annual mandatory routine rescreening of individuals who are on biologics unless there is risk for exposure.”
- Salem M, Malaty H, Hou J. The Prevalence and Characterization of Inflammatory Axial Arthropathies among Veterans with Inflammatory Bowel Disease. Gastroenterology. Jan 2018;154(1):S59.
- Bartels CM, Bell CL, Shinki K, Rosenthal A, Bridges AJ. Changing trends in serious extra-articular manifestations of rheumatoid arthritis among United States veterans over 20 years. Rheumatology (Oxford). 2010 Sep;49(9):1670-5. doi: 10.1093/rheumatology/keq135. Epub 2010 May 12.
- Pattanaik D, Gupta S, Islam S, Singhal K, Raza S. Conversion of Tuberculosis Screening Tests During Biologic Therapy Among Veteran Patient Population With Rheumatic Disease. ACR Open Rheumatol. 2019 Aug 29;1(9):542-545. doi: 10.1002/acr2.11070. eCollection 2019 Nov.