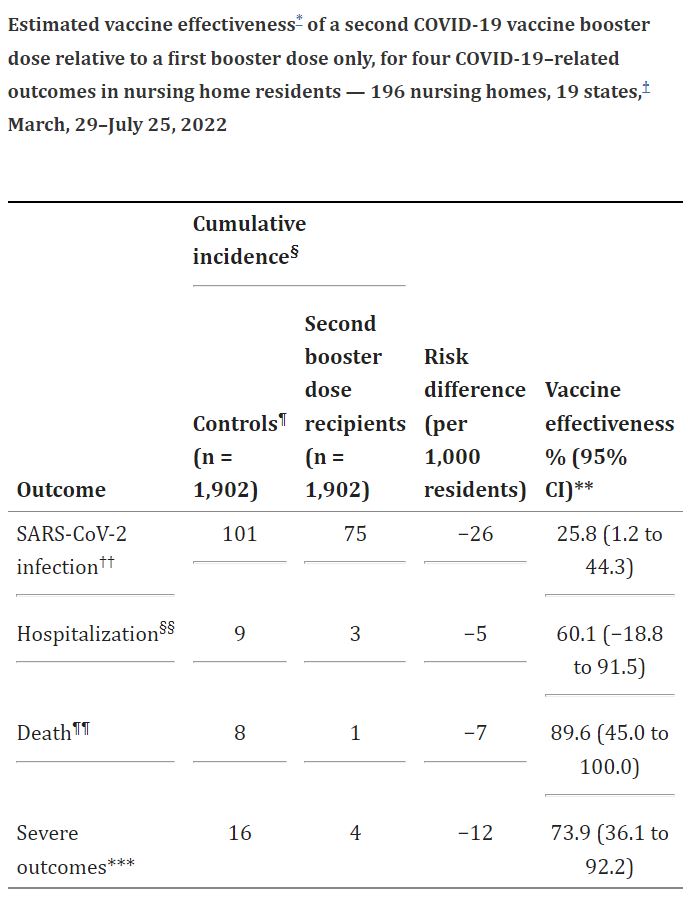
Click to Enlarge: * Through 60 days of follow-up.
† Alabama, Arizona, Colorado, Connecticut, Delaware, Kentucky, Maine, Maryland, Massachusetts, New Hampshire, New Jersey, New Mexico, North Carolina, Pennsylvania, Rhode Island, Tennessee, Vermont, Virginia, and West Virginia.
§ Events per 1,000 nursing home residents.
¶ Nursing home residents who received three previous vaccinations and were otherwise eligible to receive a second booster dose but did not receive a vaccination on a given index date during March 29–June 15, 2022.
** Bootstrapped percentile CIs.
†† Positive SARS-CoV-2 test result from antigen or reverse transcription–polymerase chain reaction testing.
§§ Transfer to acute care hospital within 21 days of a positive SARS-CoV-2 test result.
¶¶ Death within 30 days of a positive SARS-CoV-2 test result.
*** Death or hospitalization.
Source: National Library of Medicine
PROVIDENCE, RI ― Among nearly 10,000 nursing home residents in the United States included in a recent VA-led study, second mRNA COVID-19 vaccine booster doses provided significant additional protection over first booster doses against severe COVID-19 outcomes, even though the Omicron variants were emerging.
“In this analysis, comparing the relative effectiveness of a second booster dose of COVID-19 mRNA vaccines with a single booster dose among eligible nursing home residents in 19 states, VE [vaccine effectiveness] of a second booster dose against the severe composite outcomes of SARS-CoV-2–associated hospitalization or death was 73.9% and 89.6% for death alone,” according to the study led by researchers from the Center of Innovation in Long-Term Services and Supports at the Providence VAMC and Brown University. “VE against SARS-CoV-2 infection during a period crossing both Omicron subvariants BA.2 and BA.2.12.1 (March–June 2022) and BA.4 and BA.5 (July 2022) predominance was 25.8%.”
The Geriatric Research Education and Clinical Center at the Louis Stokes Cleveland VAMC also participated in the study.
Background information in the article, published in the national Center for Disease Control and Prevention’s Morbidity & Mortality Weekly Report, pointed out that nursing home residents have continued to experience significant COVID-19 morbidity and mortality compared with other U.S. cohorts.1
In March 2022, the Advisory Committee on Immunization Practices (ACIP) recommended a second mRNA COVID-19 vaccine booster dose for adults 50 and older and all immunocompromised people who had received a first booster 4 or more months earlier. More recently, on Sept. 1, 2022, ACIP voted to recommend bivalent mRNA COVID-19 vaccine boosters for all people 12 and older who had completed the primary series using monovalent vaccines two or more months earlier.
Limited Data
The authors advised that data on COVID-19 booster dose VE in the nursing home population are limited. In this analysis, academic, federal and private sector researchers evaluated routine care data collected from 196 U.S. community nursing homes to estimate VE of a second mRNA COVID-19 vaccine booster dose among nursing home residents who had received three previous COVID-19 vaccine doses—two primary series doses and one booster dose.
Residents who received second mRNA COVID-19 vaccine booster doses during March 29–June 15, 2022, with follow-up through July 25, 2022, were found to have:
- 60-day VE of 25.8% against SARS-CoV-2 (the virus that causes COVID-19 infection),
- 73.9% against severe COVID-19 outcomes (a combined endpoint of COVID-19-associated hospitalizations or deaths), and
- 89.6% against COVID-19-associated deaths alone.
“During this period, subvariants BA.2 and BA.2.12.1 (March–June 2022), and BA.4 and BA.5 (July 2022) of the B.1.1.529 and BA.2 (Omicron) variant were predominant,” the authors advised. “These findings suggest that among nursing home residents, second mRNA COVID-19 vaccine booster doses provided additional protection over first booster doses against severe COVID-19 outcomes during a time of emerging Omicron variants. Facilities should continue to ensure that nursing home residents remain up to date with COVID-19 vaccination, including bivalent vaccine booster doses, to prevent severe COVID-19 outcomes.”
Nursing home residents who had been vaccinated on a specific index date were assigned to the treatment group, while those who were unvaccinated, but eligible were assigned to the control group. Researchers determined vaccination status using residents’ immunization records from nursing home electronic health record systems.
Their analysis included 9,527 unique residents, from196 nursing homes operated by Genesis HealthCare in 19 states. Among the residents, 9,503 (99.7%) served as controls for a day or more of follow-up, and 3,245 (34.1%) residents received a second booster dose during the study period and were eligible to be included in the treatment group. The facilities were in Alabama, Arizona, Colorado, Connecticut, Delaware, Kentucky, Maine, Maryland, Massachusetts, New Hampshire, New Jersey, New Mexico, North Carolina, Pennsylvania, Rhode Island, Tennessee, Vermont, Virginia and West Virginia.
“These results indicate that, compared with a single mRNA COVID-19 vaccine booster dose, a second booster dose provided additional protection against COVID-19-associated severe outcomes among nursing home residents during the Omicron period ending with BA. 4 and BA. 5 dominances,” the researchers concluded. “The results support the importance of continued efforts to ensure the nursing home population is up-to-date on recommended COVID-19 vaccine booster doses, including the newly authorized bivalent COVID-19 vaccine.”
- McConeghy KW, White EM, Blackman C, Santostefano CM, et. al. Effectiveness of a Second COVID-19 Vaccine Booster Dose Against Infection, Hospitalization, or Death Among Nursing Home Residents—19 States, March 29-July 25, 2022. MMWR Morb Mortal Wkly Rep. 2022 Sep 30;71(39):1235-1238. doi: 10.15585/mmwr.mm7139a2. PMID: 36173757; PMCID: PMC9533729.
