FDA Rescinds Drug’s Emergency Use for COVID-19
WASHINGTON—After months of controversy on the use of hydroxychloroquine as a treatment for severe COVID-19, the Food and Drug Administration rescinded the emergency use authorization (EUA) allowing physicians to administer the drug for that purpose.
“Based on its ongoing analysis of the EAU and emerging scientific data, the FDA has determined that chloroquine and hydroxychloroquine are unlikely to be effective,” the agency said in a statement.
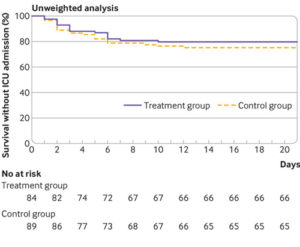
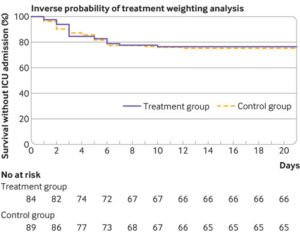
[Click image to enlarge] Kaplan-Meier curves for survival without transfer to intensive care in unweighted sample (top panel) and sample used for inverse probability of treatment weighting (bottom panel). A multivariable logistic regression model was constructed to estimate each patient’s probability of receiving hydroxychloroquine given their baseline covariates (that is, the propensity score: variables in model included age, sex, and comorbidities). ICU=intensive care unit
The move comes after initial speculation on the drug’s effectiveness, as well as growing clinical evidence that the medication and related products have no effect on COVID-19. Hydroxychloroquine is an antimalarial that is also used to treat rheumatoid arthritis, some symptoms of lupus and other autoimmune diseases.
The EAU was first granted in March after White House officials brought pressure on the drug enforcement agency to allow physicians to try hydroxychloroquine as a treatment of last resort. While recently released emails between HHS officials and FDA regulators suggest the White House was pushing for a broader EAU, FDA eventually limited the drug’s use to patients being treated in a hospital setting who were unable to register for other clinical trials.
Skepticism over the drug’s effectiveness was voiced publicly by many physicians and public health officials, but the White House continued to promote the drug as one of the few glimmers of hope for developing a treatment for those hospitalized with COVID-19. President Donald Trump later publicly lauded the drug as having “strong signs” that it would be effective against symptoms of the virus.
VA became entangled in the controversy when it took advantage of the EAU to treat COVID-19 patients with the drug. Early study results released in April showed the drug had no impact on COVID-19 patients at VA and suggested a possible higher mortality rate. VA officials were quick to note that the study, which had not yet been peer-reviewed, was a survey of existing data and that the department was not conducting a hydroxychloroquine study on veterans.1
That same month it was found that VA had spent approximately $200,000 on recent purchases of hydroxychloroquine.
As of late May, VA officials said the department was “ratcheting down” its use at VA facilities. But officials would not write off the drug as an effective treatment. In a hearing in May, VA Secretary Robert Wilkie stated that there had yet to be any double-blind, peer-reviewed studies and that the drug provided hope for patients who had none.
A study published in the British Medical Journal earlier that month, however, looked at 150 patients hospitalized for COVID-19, with half prescribed hydroxychloroquine and half receiving standard care. The study found no difference in the treatment’s impact on the disease and a higher adverse event rate in the hydroxychloroquine cohort.2
FDA officials referenced the study in their statement rescinding the EAU.
“Recent results from a large randomized clinical trial in hospitalized patients, a population similar to the population for which chloroquine and hydroxychloroquine were authorized for emergency use, demonstrated that hydroxychloroquine showed no benefit on mortality or in speeding recovery,” the agency said in its statement. “This outcome is consistent with other new data, including data showing that the suggested dosing regimens … are unlikely to kill or inhibit the virus that causes COVID-19.”
Less than a week before FDA rescinded the EAU, VA officials were still defending the drug’s potential before Congress. At a House VA Committee hearing on June 11, Richard Stone, MD, VHA’s executive-in-charge, noted that places where hydroxychloroquine was used prophylactically for malaria were experiencing a lower rate of COVID-19 hospitalization and that Brazil had “just accepted two million doses of hydroxychloroquine.”
“We believe there’s something more to this,” Stone said.
While he said that VA does not believe “that hydroxychloroquine is going to save anyone from imminent death,” the prophylactic potential of the drug had not been effectively researched.
Stone explained that VA’s $200,000 purchase of the drug in April was only partly a response to the drug’s potential as a COVID-19 treatment. The main reason for the large purchase was to ensure the department had a sufficient supply for those patients taking it for traditional reasons.
“We have more than 17,000 patients who have used hydroxychloroquine for rheumatoid arthritis or lupus. We use about 42,000 tablets … everyday,” Stone explained. “We began to find that our suppliers couldn’t supply us with the 90-day refills. I made a decision early on to go to 30-day refills to our ambulatory patients. When that supply began to loosen up, we did a block purchase to assure that our patients could be treated effectively. …The primary reason for that six million purchase was to make up for the losses of the fact that I was receiving 17-30% of my usual supply of hydroxychloroquine.”
- Magagnoli J, Narendran S, Pereira F, Cummings T, Hardin JW, Sutton SS, Ambati J. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with COVID-19. medRxiv 2020.04.16.20065920; doi: https://doi.org/10.1101/2020.04.16.20065920
- Mahévas M, Tran VT, Roumier M, et al. Clinical efficacy of hydroxychloroquine in patients with COVID-19 pneumonia who require oxygen: observational comparative study using routine care data [published correction appears in BMJ. 2020 Jun 18;369:m2328]. BMJ. 2020;369:m1844. Published 2020 May 14. doi:10.1136/bmj.m1844