VA Study in Line With Others Describing Disturbing Trend
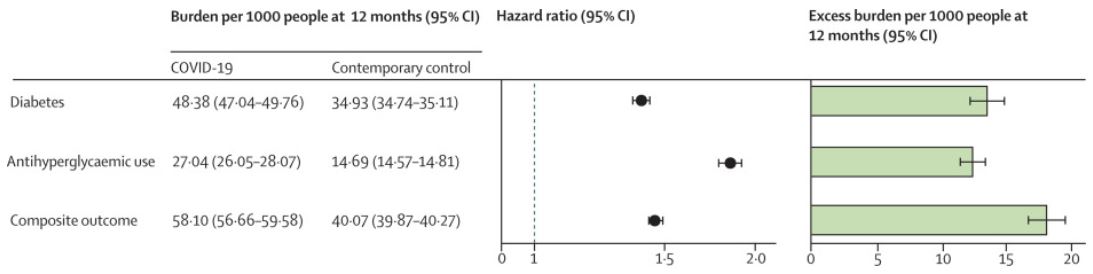
Click To Enlarge: The outcomes were ascertained from day 30 after COVID-19 infection until the end of follow-up. Adjusted hazard ratios and 95% CIs are presented in a base 10 logarithmic scale. Adjusted event rates per 1000 people at 12 months for the COVID-19 group and the contemporary control group, and the excess burden per 1000 people at 12 months and related 95% CIs are also presented.
ST. LOUIS — While it has been obvious for some time that the COVID-19 pandemic would create longer-term health effects, it is only now becoming clearer what some of those might be. Based on recent studies, including one from the VA, the development of diabetes appears to be one of the most serious of them.
“There is growing evidence suggesting that, beyond the acute phase of SARS-CoV-2 infection, people with COVID-19 could experience a wide range of post-acute sequela, including diabetes,” wrote researchers from the Clinical Epidemiology Center at the VA Saint Louis, MO, Health Care System. “However, the risks and burdens of diabetes in the post-acute phase of the disease have not yet been comprehensively characterized.”
The VA study team, Yan Xie, MPH, and Ziyad Al-Aly, MD, examined the post-acute risk and burden of incident diabetes in COVID-19 patients who survived for at least 30 days. Results were published in The Lancet Diabetes & Endocrinology.
For their cohort study, they used the VA database to build a cohort of 181,280 participants who had a positive COVID-19 test between March 1, 2020, and Sept. 30, 2021, and survived the first 30 days of COVID-19. They also created two control groups with no evidence of SARS-CoV-2 infection—a contemporary control of 4.1 million that enrolled participants during the same time period and a historical control of 4.3 million participants between March 1, 2018, and Sept 30, 2019. None of the participants had diabetes when entering the study, the authors noted.1
Over a follow-up of a median of 352 days (IQR 245-406), researchers analyzed post-acute COVID-19 risks of incident diabetes, antihyperglycemic use and a composite of the two outcomes. Two measures of risk-hazard ratio (HR) and burden per 1,000 people at 12 months were reported.
“In the post-acute phase of the disease, compared with the contemporary control group, people with COVID-19 exhibited an increased risk (HR 1·40, 95% CI 1·36-1·44) and excess burden (13·46, 95% CI 12·11-14·84, per 1,000 people at 12 months) of incident diabetes; and an increased risk (1·85, 1·78-1·92) and excess burden (12·35, 11·36-13·38) of incident antihyperglycemic use,” the study noted.
Researchers’ analysis to estimate the risk of a composite endpoint of incident diabetes or antihyperglycemic use resulted in an HR of 1·46 (95% CI 1·43-1·50) and an excess burden of 18·03 (95% CI 16·59-19·51) per 1,000 people at 12 months.
“Risks and burdens of post-acute outcomes increased in a graded fashion according to the severity of the acute phase of COVID-19 (whether patients were non-hospitalized, hospitalized, or admitted to intensive care),” the authors explained. “All the results were consistent in analysis using the historical control as the reference category.’
Researchers recommended that post-acute COVID-19 care should involve the identification and management of diabetes.
“Although diabetes and other glycometabolic abnormalities have been widely reported during the acute phase of COVID-19, less is known about the risk and burden of diabetes and related outcomes in the post-acute phase of COVID,” they added.
Another new study, led by German researchers in Düsseldorf, suggested that patients with or recovering from COVID-19 have an increased risk of developing Type 2 diabetes, but it is not known whether the effect is temporary or persists.
According to the article in Diabetologia, the human pancreas can be a target of SARS-CoV-2. That means that, following COVID-19 infection, patients can have reduced numbers of insulin secretory granules in beta cells; researchers have observed impaired glucose-stimulated insulin secretion.2
The researchers from Heinrich Heine University pointed out that some novel coronavirus patients developed insulin resistance and had elevated blood glucose levels despite no previous history of diabetes.
Those researchers advised that “the present primary care study indicates a temporal relationship between mostly mild COVID-19 and newly diagnosed Type 2 diabetes. If confirmed, this study supports the potential relevance of active monitoring of glucose dysregulation after recovery from mild forms of SARS-CoV-2 infection.”
The study team sought to quantify diabetes incidence after infection with COVID-19 by performing a retrospective cohort analysis of the Disease Analyzer, which comprises a representative panel of 1,171 physicians’ practices throughout Germany. From March 2020 to January 2021, it included 8.8 million patients.
The authors documented COVID-19 in 35,865 individuals during the study period and matched them with a control group of the same size. Participants had a mean age of 43 and were 46% female.
“Individuals with COVID-19 showed an increased Type 2 diabetes incidence compared with AURI (15.8 vs. 12.3 per 1,000 person-years),” the study notes. “Using marginal models to account for correlation of observations within matched pairs, an IRR for Type 2 diabetes of 1.28 (95% CI 1.05, 1.57) was estimated. The IRR was not increased for other forms of diabetes.”
The authors suggested, “If confirmed, these results support the active monitoring of glucose dysregulation after recovery from mild forms of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.”
The study team pointed to a number of unanswered questions for future research. Among them is whether preexisting diabetes became apparent during COVID-19 because of immunological activation or stress hyperglycemia. An investigation of whether post-COVID diabetes can be reversed after full recovery also was recommended.
In addition, researchers expressed concern about the management of new-onset diabetes in these cases, stating, “Diabetes ketoacidosis has been observed in some individuals without known diabetes even months after COVID-19. Thus, serological testing for diabetes-associated autoantibodies and C-peptide may be indicated in individuals without known risk factors for diabetes after COVID-19. Finally, the risk of hyperglycemia in individuals with COVID-19 is most likely a continuum, depending on risk factors such as injury of beta cells, an exaggerated pro-inflammatory response and changes in health behavior during the pandemic.”
- Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022 Mar 21:S2213-8587(22)00044-4. doi: 10.1016/S2213-8587(22)00044-4. Epub ahead of print. PMID: 35325624; PMCID: PMC8937253.
- Rathmann W, Kuss O, Kostev K. Incidence of newly diagnosed diabetes after Covid-19. Diabetologia. 2022 Mar 16:1–6. doi: 10.1007/s00125-022-05670-0. Epub ahead of print. PMID: 35292829; PMCID: PMC8923743.

