SINGAPORE — The worldwide prevalence of nonalcoholic fatty liver disease-related hepatocellular carcinoma is expected to increase in line with the growing obesity epidemic. NAFLD already is the fastest-growing cause of the liver cancer in the United States, France and the UK, according to a new study.
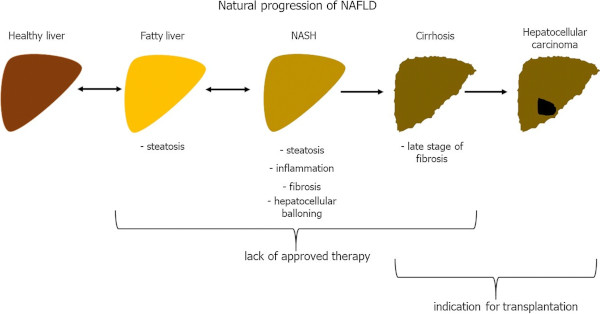
The report in Nature Reviews points out that one-fourth of the global population is estimated to have nonalcoholic fatty liver disease (NAFLD), and the incidence of nonalcoholic steatohepatitis (NASH) is predicted to increase by up to 56% in the next decade.1
NAFLD-related HCC is projected to increase substantially by 2030, with increases of 82%, 117% and 122% from 2016 in China, France and the United States, respectively, according to the article.
An international study team including participation from the Michael E. DeBakey VAMC in Houston advised that annual incidence of HCC ranges from 0.5% to 2.6% among patients with NASH cirrhosis, although the incidence of HCC among patients with noncirrhotic NAFLD is lower, estimated to be about 0.1 to 1.3 per 1,000 patient-years.
“Although the incidence of NAFLD-related HCC is lower than that of HCC of other etiologies such as hepatitis C, more people have NAFLD than other liver diseases,” the authors pointed out. “Urgent measures that increase global awareness and tackle the metabolic risk factors are necessary to reduce the impending burden of NAFLD-related HCC.”
The study explained that, based on recent research, reduced immune surveillance, increased gut inflammation and gut dysbiosis are potential key steps in tumorigenesis.
Researchers further stated that NAFLD includes simple steatosis and NASH, which can be progressive and predisposes individuals to the development of fibrosis and cancer. They also noted that NAFLD-related HCC can develop without the presence of cirrhosis.
The most important risk factor for HCC development in patients with NAFLD is diabetes, and the authors strongly recommend screening and early treatment. “Dysregulation of the gut microbiota and reduced immune surveillance are two new mechanisms that have been implicated in NAFLD hepatocarcinogenesis, and further research is warranted,” they added.
A recent study led by researchers from the Hunter Holmes McGuire VAMC and Virginia Commonwealth University, both in Richmond, also pointed out that NAFLD is closely associated with the burgeoning epidemics of diabetes and obesity. “Yet, despite NAFLD being among the most common of chronic liver diseases, the biological factors responsible for its transition from benign nonalcoholic fatty liver (NAFL) to NASH remain unclear,” the authors wrote.
The study team advised in the Journal of Lipid Resistance that the information deficit hampers the ability to find relevant animal models, predict disease progression or develop clinical treatments.2
To remedy that, they used multiple mouse models of NAFLD, human correlation data and selective gene over expression of steroidogenic acute regulatory protein (StarD1) to posit a plausible mechanistic pathway for promoting the transition from NAFL to NASH.
Results indicated that oxysterol 7α-hydroxylase (CYP7B1) controls the levels of intracellular regulatory oxysterols generated by the “acidic/alternative” pathway of cholesterol metabolism. “Specifically, we report data showing that an inability to upregulate CYP7B1, in the setting of insulin resistance, results in the accumulation of toxic intracellular cholesterol metabolites that promote inflammation and hepatocyte injury,” researchers pointed out. “This metabolic pathway, initiated and exacerbated by insulin resistance, offers insight into approaches for the treatment of NAFLD.”
The authors explained the significance of their research, summarizing that “the inability to up regulate Cyp7b1 in the setting of insufficient insulin signaling appears to establish hepatocyte accumulation of toxic intracellular cholesterol metabolites; metabolites that then initiate/perpetuate hepatocyte injury. [Findings from past research] suggest that the suppression of Cyp7b1 appears to be explained by a paucity of ‘cellular’ insulin, which under most circumstances presents as insulin resistance; a state where ‘serum’ insulin and glucagon levels are elevated. This hypothesis is further supported by the fact that focused treatment of diabetes with insulin sensitization and glucagon relief has been the most successful medicinal treatment to date. As this cellular regulatory pathway is found in most cells in the body, it may be relevant for our understanding of insulin resistance-related inflammatory pathways outside the liver that promote diseases such as atherosclerosis.”
- Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021 Apr;18(4):223-238. doi: 10.1038/s41575-020-00381-6. Epub 2020 Dec 21. PMID: 33349658; PMCID: PMC8016738.
- Kakiyama G, Marques D, Martin R, Takei H, Insulin resistance dysregulates CYP7B1 leading to oxysterol accumulation: a pathway for NAFL to NASH transition. J Lipid Res. 2020 Dec;61(12):1629-1644. doi: 10.1194/jlr.RA120000924. Epub 2020 Oct 2. PMID: 33008924; PMCID: PMC7707165.
- New therapeutic strategies in nonalcoholic fatty liver disease: a focus on promising drugs for nonalcoholic steatohepatitis. Pharmacol. Rep 72, 1–12 (2020). https://doi.org/10.1007/s43440-019-00020-1