FALLS CHURCH, VA—Predictions that the influenza vaccine would be largely ineffective in the U.S. based on results seen in Australia the previous summer troubled many federal infectious disease specialists going into last year’s flu season.
Researchers at a leading National Institute of Allergy and Infectious Diseases (NIAID) laboratory appeared contrarian, expecting the vaccine to perform about as well as usual. Based on reports from the national Centers for Disease Control and Prevention (CDC) and two DoD agencies that track vaccine effectiveness, those researchers were right. That may be cause for greater concern this year, however, flu experts suggested.
In 2017, Australia experienced record-breaking numbers of influenza-related hospitalizations and deaths. Reports indicated that the vaccine used there had at best 10% effectiveness against the dominant A strain of the virus, H3N2. According to a report in Medical Surveillance Monthly Report by DoD researchers, the vaccine’s effectiveness against H3N2 in Australia “was not statistically significantly different from zero.”1
The United States selected the same vaccine strains as Australia last year, so when H3N2 appeared likely to be the dominant circulating type here, similarly low rates of effectiveness were anticipated. While the country had the deadliest flu season in nearly a decade, the vaccine proved more protective than expected.
The CDC reported an overall vaccine effectiveness (VE) of 36%, with VE of 25% against the H3N2 strain, 67% against H1N1 and 42% against influenza B viruses.2 The H3N2 strain accounted for almost 70% of confirmed cases of influenza in the last flu season.
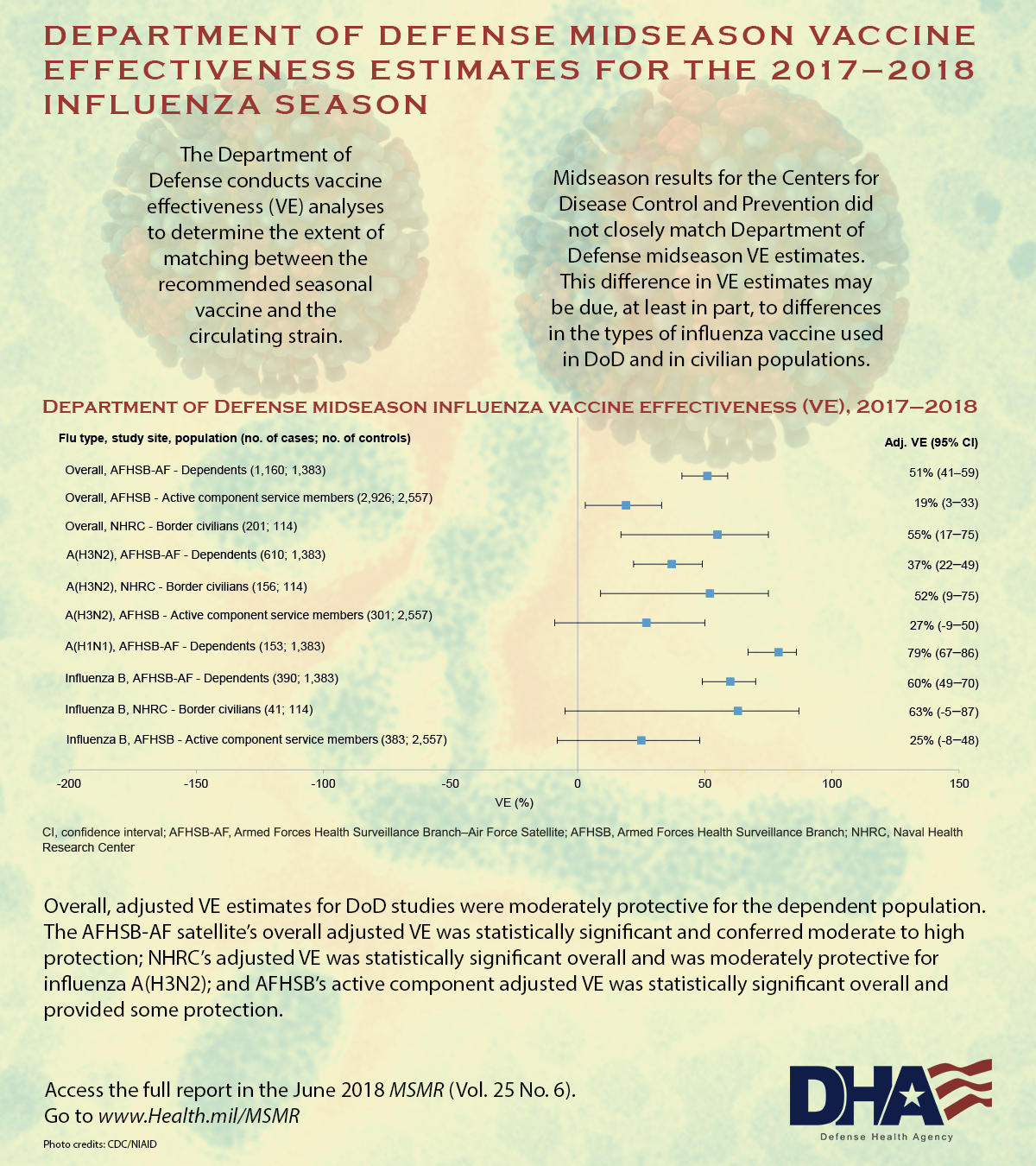
The Armed Forces Health Surveillance Branch Air Force (AFHSB-AF) found the vaccine provided substantially better protection to military dependents treated at the U.S. Air Force School of Aerospace Medicine, with an overall adjusted VE of 51% and a VE of 37% against H3N2. The Naval Health Research Center (NHRC) also determined that the vaccine had even greater effectiveness among the civilians who received care for febrile respiratory illnesses at outpatient clinics along the southwest border, with an overall adjusted VE of 55% and VE of 52% against H3N2. Based on these findings, the vaccine cut the risk of requiring medical attention for any type of influenza in half.
An AFHSB study that included only active duty servicemembers, however, found an overall VE of just 19%. The adjusted VE of 27% against the H3N2 strain was determined not to be significant as a result of low numbers and very high rates of vaccination among servicemembers (89% in cases and 90% in controls).
While the subtype analyses were generally similar, differences between the estimates of effectiveness made by the AFHSB-AF, AFHSB and the NHRC “can be attributed to the populations and sample sizes analyzed,” explained Tonya Rans, MD, chief, Immunization Healthcare Branch, Defense Health Agency. “Caution should be exercised in generalizing information from any active component VE rates to the general U.S. population,” because the high vaccination rate creates methodological issues in the analysis and repeated vaccinations might have other biologic effects.
That all the estimates had higher rates of vaccine effectiveness than predicted did not surprise Slobodan Paessler, DVM, PhD, director, Institute for Human Infections and Immunity at the Galveston National Laboratory (GNL) and professor at the University of Texas Medical Branch and member, World Health Organization Collaborating Center for Tropical Diseases. The Galveston National Laboratory is an anchor lab of the NIAID.
GNL uses a novel functional phylogenetic algorithm, the Informational Spectrum-based Phylogenetic Analysis, that “sees” viruses differently, Paessler told U.S. Medicine. “In our system, a single mutation or change in position could totally separate two viruses.” In classical phylogeny, which is used in most other labs and by the CDC, such a small change would not register as significant.
“Last year we saw two different types of H3 virus in the U.S. and Australia, and we expected the U.S. vaccine to be of average to above average effectiveness against it,” he said.
Cell Culture
Still, the DoD studies showed much greater effectiveness than the CDC reported. “This difference in VE estimates may be due, at least in part, to differences in the types of influenza vaccine used in DoD and in civilian populations,” said the MSMR authors. The majority of DoD vaccine was derived from cell culture propagation instead of egg propagation, and the DoD researchers noted that effectiveness of egg-propagated vaccine for the H3N2 strain showed a rapid decline in previous years.
A lack of effectiveness against H3N2 in egg-propagated vaccine has been attributed to a mutation generated during production, according to Paessler and his colleagues. They noted that the mutation appeared to alter the vaccine virus in a way that made in effective against a smaller fraction of the circulating viruses last year.3
While DoD’s use of cell-propagated vaccine might have improved protection last year, it will not in the coming flu season. “The Defense Logistics Agency, which is responsible for contracting the DoD supply of influenza vaccines, purchased all egg-propagated products” this year, Rans said, noting that the Advisory Committee on Immunization Practices did not express a preference for any influenza vaccine product for 2018-2019.
In general, Rans said the vaccine selected should be a good match for the influenza viruses expected in the coming months. “It is difficult to predict VE or circulating influenza strains from year to year, which is why influenza surveillance is conducted year-round. Currently, we are not seeing circulating strains that raise suspicion of a poor match with vaccine strains.”
Paessler again took a contrarian view. He said he expects the 2018-2019 influenza vaccine to be less effective than the 2017-2018 vaccine. In fact, “we should use last year’s,” he said. The new vaccine anticipated a shift in the H3 virus, based on the Australian experience, but the same changes have not occurred in the United States.
He suggested that the U.S. will have a very different influenza this winter than the Southern Hemisphere has experienced the last few months. About 90% of cases reported so far in Australia have been of the H1N1 type “and the match is pretty good for that,” Paessler said.
Australia has had very little H3 activity, and labs there deposited an “extremely low” number of H3 sequences in GISAID EpiFlu Database, which organizations worldwide use to share and study influenza viruses. U.S. labs have already deposited more than 300 H3 sequences, Paessler noted.
A GNL analysis determined that the new vaccine for H3N2 is compatible with just 8.8% of the isolates found in the U.S. earlier this year. Last year’s vaccine had a 71% compatibility with H3N2 viruses circulating in early 2017. The low compatibility suggests that “VE for the next flu season in U.S. could be very low,” Paessler said. The analysis found that last year’s vaccine, which had the H3N2 virus A/Hong Kong/4801/2014, would work better than the current vaccine, which has H3N2 virus A/Singapore/INFIMH-16-0019/2016.
Whatever strain dominates this season, Paessler stressed the importance of getting a flu shot. The vaccine is a good match for H1 strains and a “pretty good match” for influenza B strains and could keep those who do contract influenza from developing more serious complications.
1. Shoubaki L, Eick-Cost AA, Hawksworth A, Hu Z, Lynch L, Myers CA, Federinko S.Brief report: Department of Defense midseason vaccine effectiveness estimates for the 2017-2018 influenza season. MSMR. 2018 Jun;25(6):26-28.
2. Flannery B, Chung JR, Belongia EA, McLean HQ, Gaglani M, Murthy K, Zimmerman RK, Nowalk MP, Jackson ML, Jackson LA, Monto AS, Martin ET, Foust A, Sessions W,Berman L, Barnes JR, Spencer S, Fry AM. Interim Estimates of 2017-18 Seasonal Influenza Vaccine Effectiveness-United States, February 2018. MMWR Morb Mortal Wkly Rep. 2018 Feb 16;67(6):180-185.
3. Paessler S, Veljkovic V. Prediction of influenza vaccine effectiveness for the influenza season 2017/18 in the US. F1000Res. 2017 Nov 29;6:2067
4. Paessler S, Veljkovic V. Using electronic biology based platform to predict flu vaccine efficacy for 2018/2019. F1000Research. 2018;7:298. doi:10.12688/f1000research.14140.2.
