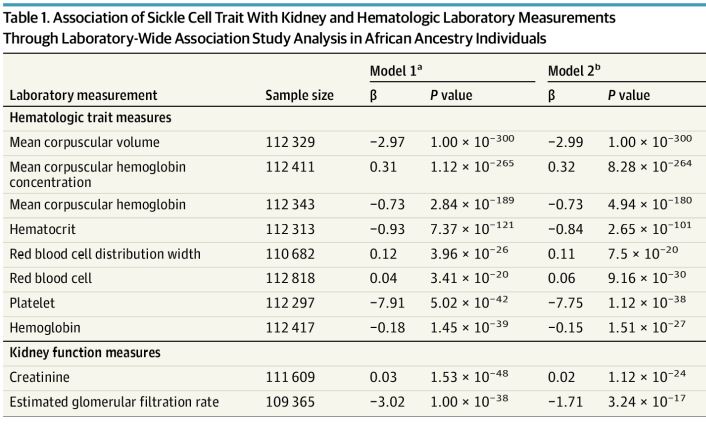
Click to Enlarge: Association of Sickle Cell Trait With Kidney and Hematologic Laboratory Measurements Through Laboratory-Wide Association Study Analysis in African Ancestry Individuals a) Model 1: Models adjusted by sex, age, age squared, and first 20 principal components.
b) Model 2: Models adjust by sex, age, age squared, first 20 principal components, and chronic kidney disease. Source: JAMA Internal Medicine
PHILADELPHIA — The presence of sickle cell trait (SCT) should be considered an adverse prognostic factor for COVID-19, according to a new study.
The genetic association study of 2,729 adults with SCT and 129 848 who were SCT negative, published in JAMA Internal Medicine, determined that SCT patients had a range of preexisting kidney conditions that were associated with unfavorable outcomes following infection with SARS-CoV-2. The most serious was an increased risk of mortality and acute kidney failure following COVID-19, according to researchers from the University of Pennsylvania Department of Medicine and the Corporal Michael J. Crescenz VA Medical Center, both in Philadelphia, and colleagues.
VA researchers from West Haven, CT; Durham, NC; Phoenix; Salt Lake City; Portland, OR; and San Francisco also participated in the study.
SCT is defined as the presence of 1 hemoglobin beta sickle allele (rs334-T) and 1 normal beta allele. It affects millions in the United States, especially those of African and Hispanic ancestry. Until now, however, the association of SCT with COVID-19 has been unclear.
The study team sought to assess the association of SCT with the pre-pandemic health conditions in participants of the Million Veteran Program (MVP), including an analysis of the severity and sequelae of COVID-19.
To do that, researchers focused on COVID-19 clinical data of 2,729 SCT patients, of whom 353 had COVID-19, as well as, 129,848 SCT-negative individuals, of whom 13 488 had COVID-19. Data for the study were collected between March 2020 and February 2021.
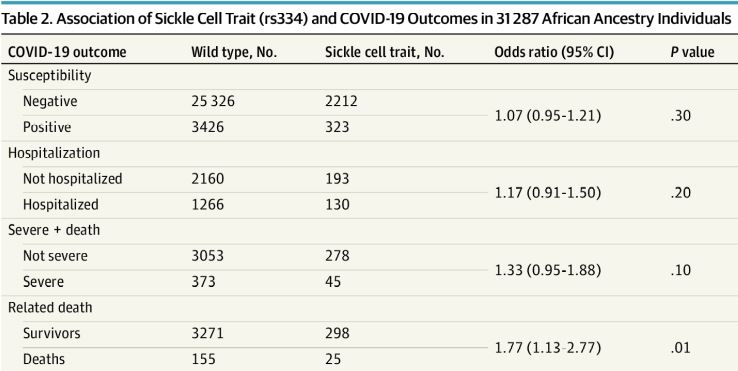
Click to Enlarge: Association of Sickle Cell Trait (rs334) and COVID-19 Outcomes in 31 287 African Ancestry Individuals Source: JAMA Internal Medicine
Of the 132, 577 MVP participants with COVID-19 data, mean (SD) age at the index date was 64.8 (13.1) years, according to the authors, who advise that sickle cell trait was present in 7.8% of individuals of African ancestry and associated with a history of chronic kidney disease, diabetic kidney disease, hypertensive kidney disease, pulmonary embolism and cerebrovascular disease.
SCT was found to be linked to increased COVID-19 mortality in individuals of African ancestry (n = 3,749; odds ratio, 1.77; 95% CI, 1.13 to 2.77; P = 0.01). In the 60 days following COVID-19 and an increased incidence of acute kidney failure. “A counterfactual mediation framework estimated that on average, 20.7% (95% CI, -3.8% to 56.0%) of the total effect of SCT on COVID-19 fatalities was due to acute kidney failure,” the researchers point out, adding, “In this genetic association study, SCT was associated with preexisting kidney comorbidities, increased COVID-19 mortality and kidney morbidity”.
Background information in the study stated that the US incidence estimate for SCT was 73.1 cases per 1,000 Black newborns, 6.9 cases per 1,l000 Hispanic newborns and 3.0 cases per 1,000 white newborns.
Increased Risks
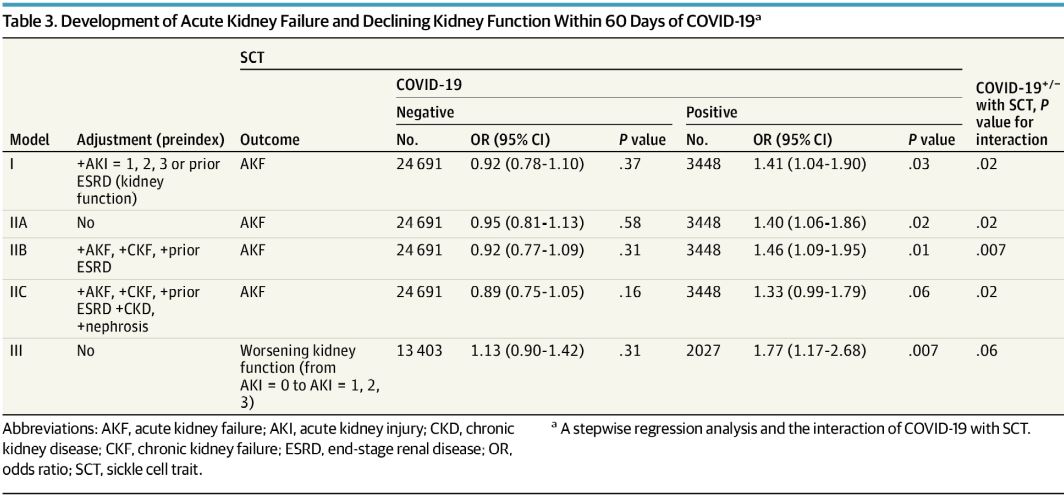
Click to Enlarge: Development of Acute Kidney Failure and Declining Kidney Function Within 60 Days of COVID-19 Source: JAMA Internal Medicine
“Although largely considered a benign condition, SCT has been associated with increased risk for adverse outcomes ranging from rare complications of exertion-related injuries and renal medullary carcinoma to more common medical conditions such as chronic kidney disease and venous thromboembolism,” the authors explain.
They added, “The Centers for Disease Control and Prevention (CDC) has advised that patients with SCD be regarded as highly susceptible to COVID-19. However, this cautionary advice does not extend to individuals with SCT.”
Even though sickle cell trait affects more than 3 million people in the US and 300 million people globally, limited data exist on the association between SCT and COVID-19 outcomes. Researchers said they used the Million Veteran Program (MVP) data because it has clinical information pre-COVID-19 and post-COVID-19 for SCT in more than 658 582 veterans.
The study team was able to demonstrate that people with SCT were predisposed to multisystem alterations, especially kidney disease. “Multiple correlated chronic kidney conditions derived from the EHR were associated with SCT, corroborated by a decrease in median laboratory values for estimated glomerular filtration rate and elevation in the baseline creatinine level,” the article noted. “Veterans of African ancestry (n = 123,120) with preexisting kidney codes or displaying signs of the multisystem disorder showed significant association with COVID-19 death.”
Researchers also discovered a significant association between SCT, and a diagnosis of acute kidney failure within 60 days of COVID-19. “The increased risk of AKF persisted despite adjustment for preexisting kidney conditions or declining kidney function based on laboratory values,” they explained. “These observations indicated that pre-COVID-19 kidney impairment only explained a small fraction of increased risk for post-COVID-19 AKF, suggesting that the mechanisms for AKF might be different for individuals with SCT and COVID-19.”
That led to the observation that the association of SCT with kidney dysfunction—before and after COVID-19—suggests an active role of sickle hemoglobin in the pathogenesis of kidney function abnormalities. In fact, researchers calculated that an AKF diagnosis within 60 days of COVID-19 was the cause for more than 20% of COVID-19 deaths in veterans with African ancestry with SCT. “In summary,” they wrote. “There was an increased risk of death from COVID-19 among SCT carriers.”
Background information in the study noted that chronic kidney disease, with a prevalence of 3% to 13%, was one of the most common comorbidities in the hospitalized patients with COVID-19. Others were:
- hypertension (48%-57%),
- diabetes (17%-34%),
- cardiovascular disease (21%-28%),
- chronic pulmonary disease (4%-10%), and
- malignant neoplasm (6%-8%).
Previous studies have linked those conditions to severe COVID-19 outcomes. The authors posited, “The polymerization of hemoglobin beta sickle protein in SCD contributes to vaso-occlusion. In individuals with SCT, sickling due to low oxygenation tension in the kidneys may cause kidney dysfunction, which can be exacerbated by COVID-19, in addition to other potential mechanisms. Of note, gout has a known association with SCD, but its linkage to SCT identified through association studies has not been previously reported.”
Earlier studies on the association of SCT and COVID-19 outcome were much smaller and unable to reach the conclusions of this one.
“Our findings suggest that SCT can further contribute to worse outcomes in individuals of African ancestry, and there is a need for new treatment strategies to improve clinical outcomes of COVID-19 in individuals with SCT,” researchers wrote, adding, “The presence of an HbC allele was not associated with worse COVID-19 outcomes. The lack of associations of HbC with multiple medical/kidney comorbidities may explain the difference in COVID-19 outcomes.”
Long before COVID-19, the US military has been grappling with the ramifications of sickle cell trait and disease for half a century. In 2020, the Army joined the other services in testing recruits for sickle cell trait.
Over the years, sickle cell train has been implicated in deaths during training exercises at elevation, including four Marine recruits in 1968. More recently, SCT was found to be a contributing factor in the death of an Eglin Air Force Base captain after a fitness test in August 2019.

