JACKSONVILLE, FL — Suboptimal adherence to new therapies for chronic lymphocytic leukemia (CLL)/small lymphocytic leukemia (SLL), as well as related costs and healthcare resource use, demonstrate unmet needs in real-world treatment of the blood cancers, according to a new review.
CLL and related SLL are the most common types of leukemia in adults in the United States. The U.S. veteran population, predominantly older males, are at high risk for CLL/SLL, particularly if they had exposure to Agent Orange or other herbicides during military service.
In the past decade, there have been a number of advances in the treatment of CLL/SLL. Novel, oral targeted therapies—chiefly inhibitors of Bruton tyrosine kinase (BTK) (e.g. ibrutinib) and apoptosis regulator B-cell leukemia/lymphoma 2 (BCL-2) (e.g. venetoclax)—have increasingly replaced chemotherapy for CLL/SLL. Often given in combination with anti-CD20 monoclonal antibodies (e.g., rituximab, ofatumumab or obinutuzumab), these therapies have been associated with increased survival. Yet little is known about the burden of CLL/SLL in veterans, or the clinical or economic outcomes of current treatment.
The findings of a new study provide insight, highlighting the clinical and economic burden of the disease, as well as the real-world unmet needs of CLL/SLL among the veteran population.1
In the retrospective observational study, sponsored by the industry, researchers at the Mayo Clinic in Jacksonville, FL, identified adults newly diagnosed with CLL/SLL in the VHA dataset from October 2014 to September 2019. Eligible patients were required to have at least two visits with CLL/SLL diagnosis, and continuous enrollment of six months pre- and three months post-index date, defined by the first diagnosis date.
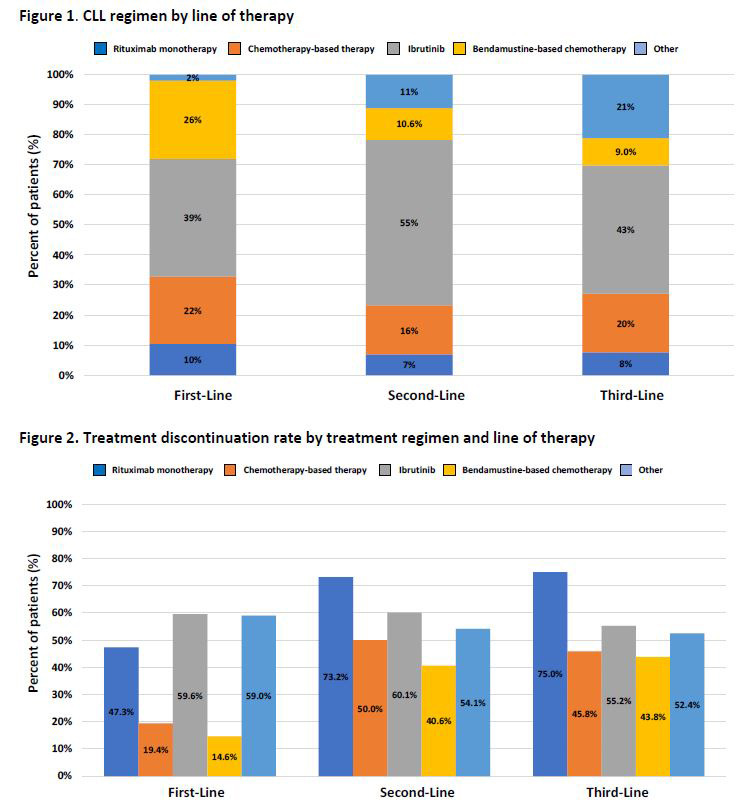
The study population was further required to have at least one CLL/SLL treatment on or after the index date and no CLL/SLL treatment anytime prior to the index date. Treatment regimens were identified by line of therapy and categorized into five mutually exclusive categories: bendamustine-based (alone or in combination) therapy, other chemotherapies, ibrutinib, rituximab-monotherapy or other regimens. Adherence—as measured by discontinuation and switching rates—and economic outcomes, as measured by healthcare resource utilization and costs—were also assessed.
The researchers examined costs and healthcare resource utilization by first-, second- and third-line therapy. Healthcare resource utilization examined included hospitalization and length-of-stay. Total costs were reported for all-cause and CLL/SLL-related, and calculated as the sum of inpatient, outpatient and pharmacy costs per-patient-per-month.
Key findings included:
Patient characteristics: Of the 13,664 veteran patients diagnosed with CLL/SLL, 79% were in watch-and-wait. The final study population consisted of 2,861 patients who received one or more CLL/SLL therapies. Most patients were elderly (median age 70 years), white (83%) and male (98%). At baseline, approximately 39% of the veterans had concurrent use of proton pump inhibitors.
Treatment patterns: The average time to treatment initiation, from diagnosis to first-line therapy, was 315 days. Just more than one-fourth (26.9%) of patients who received first-line therapy further received second-line therapy, with a mean duration of 318 days of treatment. Only 7% received third-line therapy, with a mean duration of treatment of 229 days.
Treatment discontinuation: Overall, treatment discontinuation rates were high across current regimens in each line of therapy, with 73% (mean duration 354 days) for first-line; 66% (mean duration: 241 days) for second-line and 59% (mean duration 190 days) for third-line. Discontinuation rates were generally highest for bendamustine-based chemotherapies, with 85%, 84% and 89% discontinuing in first-line, second-line and third-line, respectively. Ibrutinib accounted for over half of total patient discontinuations, with discontinuation rates at 60%, 60% and 55% in first-line, second-line and third-line, respectively.
Healthcare Resource Utilization and Costs: The CLL/SLL-related hospitalization rate was 39% with an average length of stay of seven days. Total per-patient-per-month all-cause and CLL/SLL-related costs were $26,709 and $17,233, respectively; these costs were increased by line of therapy. Controlling for patient clinical and demographic covariates, treatment discontinuation and
treatment switching were statistically significant predictors of higher inpatient admissions and LOS of hospitalizations
“This real-world data demonstrated significant clinical and economic burden associated with CLL/SLL among the U.S. veterans,” the authors concluded. “Furthermore, the suboptimal adherence, as reported by high treatment discontinuation rates and its impact on increasing costs and healthcare resource use, highlights the real-world unmet needs of CLL/SLL management in the veteran population,” they wrote. “Future studies are needed to further understand the long-term outcomes of each treatment regimen.”
- Yang K, Liu S, Tang B, Chanan-Khan A. Real-World Treatment Patterns, Adherence and Healthcare Resource Utilization for Chronic Lymphocytic Leukemia/Small Lymphocytic Leukemia Among Veterans in the United States. Blood, Volume 138, Supplement 1, 2021, Page 4079, ISSN 0006-4971. https://doi.org/10.1182/blood-2021-148716.