VA Data Helps Show How Patients Fared With Coronavirus Infection
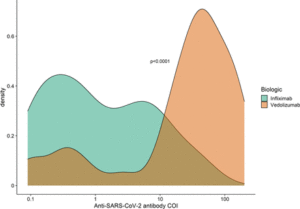
Density plot of the magnitude of anti-SARS-CoV-2 antibody reactivity stratified by biological therapy among participants who had a positive PCR to anti-SARS-CoV-2 at least 2 weeks prior to their serology sample. COI, cut-off index.
OXFORD, UK – Patients with autoimmune diseases had significantly worse outcomes with COVID-19 infection than with past cases of influenza, according to a review using data from the VA and other international data.
What is especially concerning is evidence mounting that some of the most effective drugs used to treat auto-immune ailments also might make COVID-19 vaccines less effective.
A report in the journal Rheumatology (Oxford) were cautioned to carefully follow stay-at-home advisories to avoid COVID-19, but not much information was available initial on how they would far with infection. To remedy that, a University of Oxford-led study team characterized 30-day outcomes and mortality after hospitalization with COVID-19 among patients with prevalent autoimmune diseases, comparing outcomes after hospital admissions among similar patients with seasonal influenza.1
The multinational network cohort study was conducted using electronic health records data from Columbia University Irving Medical Center, Optum, IQVIA claims data and the VA data system in the United States, as well as Information System for Research in Primary Care-Hospitalization Linked Data in Spain and claims data from Health Insurance and Review Assessment in South Korea. Included were all patients with prevalent autoimmune diseases, diagnosed and/or hospitalized between January and June 2020 with COVID-19, and similar patients hospitalized with influenza in 2017-2018. Defined as outcomes were death and complications within 30 days of hospitalization.
The focus was on 133,589 patients diagnosed and 48,418 hospitalized with COVID-19 with prevalent autoimmune diseases. Most patients were female and 50 or younger with previous comorbidities.
The authors pointed out that the prevalence of hypertension (45.5-93.2%), chronic kidney disease (14.0-52.7%) and heart disease (29.0-83.8%) was higher in hospitalized vs diagnosed patients with COVID-19. Results indicated that, compared with 70,660 patients hospitalized with influenza, those admitted with COVID-19 had more respiratory complications including pneumonia and acute respiratory distress syndrome, and higher 30-day mortality (2.2% to 4.3% vs 6.3% to 24.6%).
“Compared with influenza, COVID-19 is a more severe disease, leading to more complications and higher mortality,” researchers concluded.
Meanwhile, another UK study involving inflammatory bowel disease (IBD) patients called into question the effectiveness of COVID-19 vaccines in people prescribed anti-tumor necrosis factor (anti-TNF) drugs,
An online report in the journal Gut discussed how infliximab, commonly prescribed to Crohn’s disease and ulcerative colitis patients, blunts the immune system to COVID-19 infection, potentially increasing the risk of reinfection.2
That information comes from the CLARITY study, which recruited 6,935 patients with Crohn’s disease and ulcerative colitis from 92 UK hospitals between September and December 2020. It determined that fewer than half of people with IBD who were treated with infliximab had detectable antibodies after SARS-CoV-2 infection, the coronavirus that causes COVID-19.
Authors of that study warned that an impaired immune response might boost susceptibility to recurrent COVID-19 and even help drive the evolution of new variants of SARS-CoV-2, the virus responsible for the infection. Still, they encouraged patients to continue to take their anti-TNF medications because overall Covid-19 risk remains very low.
Researchers also strongly advised close monitoring of IBD patients treated with infliximab, who have been vaccinated against COVD-19. The concern is their ability to mount a strong enough antibody response to be protective against infection, even after immunization.
CLARITY study lead, Professor Tariq Ahmad, DPhil, of the University of Exeter Medical School, said: “The poor antibody responses observed in patients treated with infliximab raise the possibility that some patients may not develop protective immunity after COVID-19 infection, and might be at increased risk of reinfection,” explained lead researcher Tariq Ahmad of the University of Exeter Medical School. “What we don’t yet know is how use of anti-TNF drugs will impact antibody responses to vaccination.”
Ahmad said the study “will continue to follow participants for 40 weeks to investigate important questions regarding the impact of immunosuppressive drugs on immunity to SARS-CoV-2 infection and COVID-19. Modified vaccine schedules may be required if impaired antibody responses are also observed following vaccination. However, because the overall risk of COVID-19 is low in this patient group, we would still strongly encourage patients to continue to take anti-TNF medicines.”
Background information in the study advises that about 2 million patients worldwide are prescribed anti-TNF drugs, which include infliximab and are effective treatments for immune-mediated inflammatory diseases. The downside in the current pandemic, however, is that the drugs also suppress the immune system, thereby reducing vaccine effectiveness and increasing risk of serious infection.
For the CLARITY study, researchers compared antibody responses to SARS-CoV-2 in patients treated with infliximab to an alternative medication, vedolizumab, that blocks inflammatory cells entering the gut without reducing immune responses to infections or vaccinations. To all, they recruited 6,935 IBD patients, average age 39, were recruited from 92 UK hospitals between September and December 2020. About two thirds of them were being treated with infliximab, with the remaining on vedolizumab.
About 8% of the infliximab group and 9% of the vedolizumab group had symptoms indicative of COVID-19 infection, and 89 of those taking infliximab and 38 of those taking vedolizumab tested positive for the virus. .
Results indicated that, while rates of COVID-19 infection and hospitalizations were similar between infliximab- and vedolizumab-treated patients, infliximab-treated patients who recovered were much less likely to have a positive antibody test – 3.4% for infliximab vs. 6% for vedolizumab.
Only about half, 48%, of patients treated with infliximab whose COVID-19 infection was confirmed by a swab test subsequently developed antibodies compared to 83% of those treated with vedolizumab, according to researchers.
The authors suggested the findings couldn’t be explained by differences in acquisition or severity of infection alone, but that infliximab appeared to be directly influencing antibody responses to infection. Supporting that supposition was that rates of positive antibody tests were lowest in participants who were also taking other drugs that suppress the immune system, such as azathioprine, mercaptopurine or methotrexate.
- Tan EH, Sena AG, Prats-Uribe A, You SC, et. Al. COVID-19 in patients with autoimmune diseases: characteristics and outcomes in a multinational network of cohorts across three countries. Rheumatology (Oxford). 2021 Mar 16:keab250. doi: 10.1093/rheumatology/keab250. Epub ahead of print. PMID: 33725121.
- Kennedy NA, Goodhand JR, Bewshea C, Nice R, et. Al. Anti-SARS-CoV-2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut. 2021 Mar 22:gutjnl-2021-324388. doi: 10.1136/gutjnl-2021-324388. Epub ahead of print. PMID: 33753421; PMCID: PMC7992387.
