
Sherrie Aspinall, PharmD, MSc, national program manager of the VA Center for Medication Safety/VA Pharmacy Benefits Management Services
Each year, approximately 2 million serious adverse drug reactions (ADRs) occur in the U.S. and are responsible for around 100,000 deaths, according to the Food and Drug Administration’s Center for Drug Evaluation and Research.
ADRs account for 20% of the injuries occurring in hospitals, and they double the length of stay and the cost of hospitalization. Nearly 7% of the generalized hospital population in the United States experience ADRs.
A new study, which is the first to examine the cost of serious ADRs to the VHA, provides information that may be used by decision-makers to estimate the cost avoidance of interventions to reduce them, according to the study’s authors.
“Since approximately 2007 the VHA has spontaneously reported adverse drugs reactions in a national database; however, we never attempted to assign a cost to the VHA of these ADRs,” said Sherrie Aspinall, PharmD, MSc, national program manager of the VA Center for Medication Safety/VA Pharmacy Benefits Management Services. Although other studies have examined the cost of ADRs resulting in hospitalization, differences in population/setting and medications limited the generalizability of the findings to VHA, she said. In addition, information about costs by medication or drug-symptom pair – for example, lisinopril-angioedema — was minimal.
In the new study, published in Pharmacy and Clinical Pharmacology, Aspinall and her colleagues sought to estimate total medical costs for spontaneously reported severe ADRs by drug-symptom pair that resulted in or contributed to hospitalizations.1
Using the national database of spontaneously reported ADRs, they extracted those ADRs that occurred in outpatients and resulted in hospitalization during fiscal years (FYs) 2014 to 2018. They then linked those reports with VHA acute care hospitalizations near the date of the ADR using data from the VHA Health Economic Resource Center. “These data also include the cost of the hospitalizations. Therefore, the costs they used are specific for the hospitalizations of the patients who experienced the ADRs,” said Aspinwall, who is on staff at the VA Pittsburgh Healthcare System and an associate professor in the School of Pharmacy at the University of Pittsburgh.
The study included 5,113 outpatients’ ADR reports from 4880 veterans. The mean patient age was 67.7 years; most patients were male and white. A total of 2,792 of the 5,113 outpatients’ ADR reports (54.6%) that resulted in or contributed to VHA hospitalizations included one symptom, and, 3918 (76.6%) involved one medication.
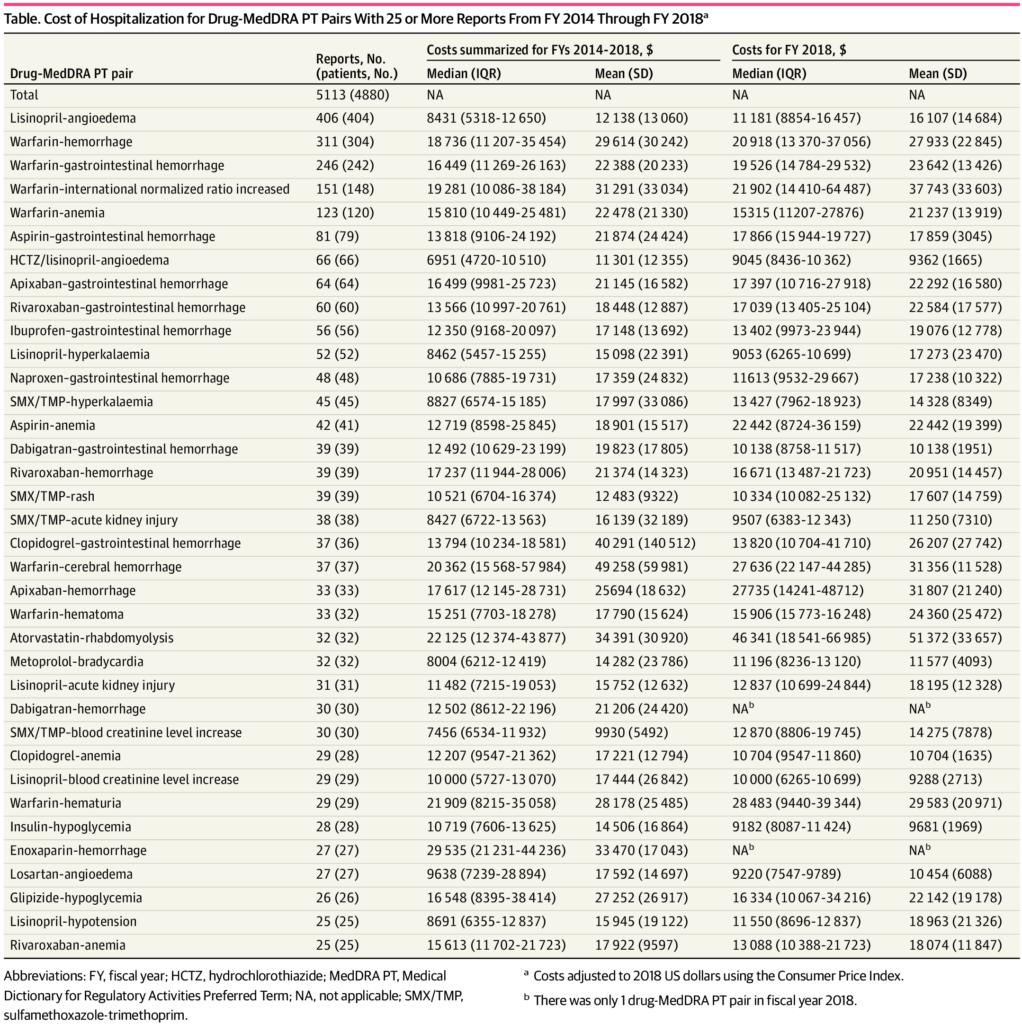
Click to Enlarge: Cost of Hospitalization for Drug-MedDRA PT Pairs With 25 or More Reports From FY 2014 Through FY 2018 Source: JAMA Network
Most Reported
In total, the researchers identified 36 drug-symptom pairs that had 25 or more reports. The most-reported drug-symptom pairs were lisinopril-angioedema (406 reports [7.9%]), warfarin-hemorrhage (311 [6.1%]), and warfarin–gastrointestinal hemorrhage (246 [4.8%]).
Hydrochlorothiazide/lisinopril-angioedema had the lowest median cost during fiscal years 2014 through 2018 at $6951 (interquartile range [IQR], $4720-$10,510). Enoxaparin-hemorrhage had the highest median cost at $29 535 (IQR, $21 231-$44,236).
Costs of other ADRs with 25 or more reports, all adjusted to 2018 US dollars included: warfarin-hemorrhage, $18,736 (IQR, $11,207 – $35,454), warfarin-cerebral hemorrhage, $20,362 (IQR, $15,568-57,984), atorvastatin-rhabdomyolysis, $22,125 (IQR, $12,374 – $43,877) and losartan-angioedema, $9638 (IQR $7239-$28,894).
“Regardless of the medication involved or the adverse event, hospitalizations due to ADRs are costly,” said Aspinall. At the drug-symptom level, the investigators found potential cost differences for the same adverse reaction due to different medications. For example, hospitalization for gastrointestinal hemorrhage with warfarin is more costly than gastrointestinal hemorrhage with rivaroxaban or dabigatran.
Aspinall said this information can be used to describe the cost of specific, severe ADRs more accurately and could be used to estimate the cost avoidance of interventions to reduce ADRs, for example with a newly developed oral anticoagulant dashboard.
She said the group is now reviewing additional data they collected as a part of the study to examine the clinical and policy implications of their findings.
- Aspinall SL, Vu M, Moore V, Jiang R, Au A, Bounthavong M, Glassman PA. Estimated Costs of Severe Adverse Drug Reactions Resulting in Hospitalization in the Veterans Health Administration. JAMA Netw Open. 2022 Feb 1;5(2):e2147909. doi: 10.1001/jamanetworkopen.2021.47909. PMID: 35142836; PMCID: PMC8832171.

