Until last fall, no established standard of care existed for newly diagnosed peripheral T-cell lymphoma. But, using a new process, the Food and Drug Administration agreed to expand the indications for brentuximab vedotin only two weeks after receiving an application. Among the keys to successful use of the new treatment are subtype and CD30 testing, according to the VA, which treats more patients with rare conditions such as PTCL than any other healthcare system.
DURHAM, NC—In near record-setting speed, the U.S. Food and Drug Administration approved brentuximab vedotin, the first treatment for newly diagnosed peripheral T-cell lymphoma, only two weeks after receiving the application for the expanded indication.
The FDA granted breakthrough therapy designation and priority review to the supplemental application and approved it under the Real-Time Oncology Review Pilot Program the following day. The pilot program was launched last summer to expedite access to potentially life-saving cancer treatments that appear likely to provide substantial improvements over existing treatments based on top-line results.
That was clearly the case for the monoclonal antibody brentuximab vedotin in PTCL. PTCL is an aggressive malignancy with poor long-term survival. Until the approval of brentuximab, there was no established standard of care for the rare disease.
“This is the first U.S. Food and Drug Administration approval for treatment of patients with newly diagnosed peripheral T‐cell lymphomas (PTCL). Improvement in progression‐free and overall survival over cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy, which has been the standard of care for decades, is unprecedented,” wrote the authors of a recent review in The Oncologist. “The new regimen represents a major advance for the frontline treatment of patients with CD30‐expressing PTCL.”1
As the nation’s largest integrated health system, the VA treats more patients with rare conditions than any other healthcare system in the United States. And no medical provider has more experience with uncommon diseases that particularly affect older men, such as peripheral T-cell lymphoma.
“PTCL is a term that encompasses many different types of T-cell lymphomas, all of which are very rare in the U.S. and the treatments offered depends on the subtype and CD30 testing (by immunostain),” said Daphne R. Friedman, MD, staff physician at the Durham, NC, VAMC.
PTCL comprises between 5% and 10% of all non-Hodgkin lymphomas and includes anaplastic large cell lymphoma, angioimmunoblastic T-cell lymphoma, extranodal natural killer/T-cell lymphoma, T-cell prolymphocytic leukemia, hepatosplenic T-cell lymphoma, PTCL-not otherwise specified and others.
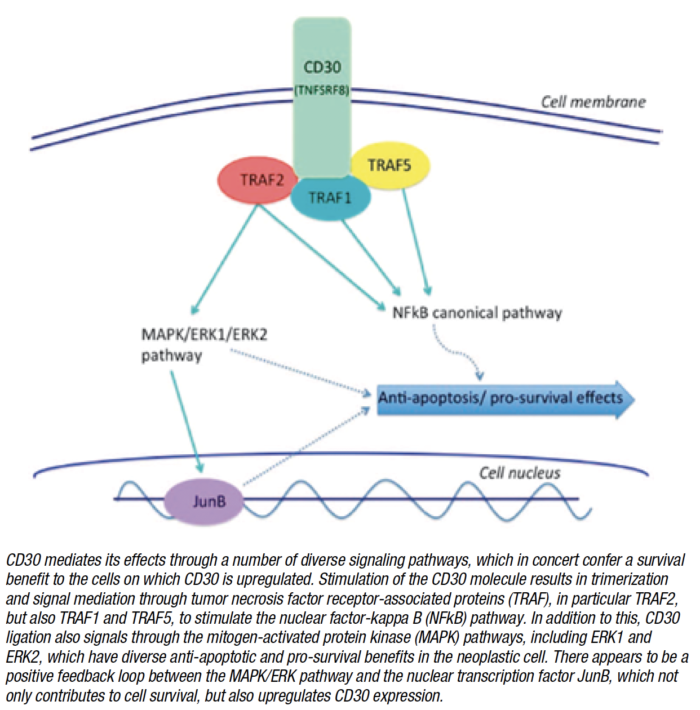
About half of PTCLs express CD30, a protein found on the surface of certain cells. A number of hematological malignancies characteristically express CD30, including anaplastic large cell lymphoma and Hodgkin lymphoma and several other types of PTCL and some B-cell non-Hodgkin lymphomas express variable degrees of CD30.
The FDA approved brentuximab vedotin in combination with chemotherapy for previously untreated systemic anaplastic large cell lymphoma (sALCL) or other CD30-expressing peripheral T-cell lymphomas, including angioimmunoblastic T-cell lymphoma and PTCL not otherwise specified. This was the first FDA approval for previously untreated PTCL including sALCL.
The National Comprehensive Cancer Network and other guidelines have recommended a variety of therapies for PTCL. “Examples of chemotherapy that may be considered are CHOP, CHOEP, CHP-brentuximab, single agent brentuximab, and pralatrexate, and sometimes autologous stem cell transplant is incorporated as part of therapy,” Friedman told U.S. Medicine.
“The current standard of care for initial treatment of peripheral T-cell lymphoma is multiagent chemotherapy. That treatment has not significantly changed in decades and is too often unsuccessful in leading to long-term remissions, underscoring the need for new treatments,” explained Steven Horwitz, MD, Department of Medicine, Lymphoma Service, Memorial Sloan Kettering Cancer Center, NY, lead author of the study on which the FDA based its approval.
Previous studies showed that patients who received cyclophosphamide, doxorubicin, vincristine and prednisone achieved better outcomes than patients on other chemotherapy regimens, but five-year survival has remained at 32% to 43% for most subtypes.2
The Phase III ECHELON-2 study which led to the expanded approval for brentuximab enrolled 452 patients with CD30-expressing PTCLs to either CHOP or cyclophosphamide, doxorubicin and prednisone plus brentuximab.3 More than 140 institutions participated in the study.
Progression-Free Survival
At 36 months, the trial showed that the brentuximab combination more than doubled progression-free survival compared to CHOP, to 48 months from 20.8 months, with three-year progression-free survival of 57% compared to 44% for CHOP.
In ALK-positive systemic ALCL, which universally expresses CD30, the brentuximab combination reduced the risk of progression, death or change of treatment by 71%. For patients who proceeded to stem cell transplant, the improvement in progression free survival was 29%.
With a median follow up of 42 months, median overall survival had not been met in the brentuximab combination arm and was 17.5 months in the CHOP arm.
More patients responded to the brentuximab combination compared to CHOP, 83% vs. 72%, and a higher percentage experienced complete response, 68% compared to 56%.
Both therapies had similar safety profiles.
“The ECHELON-2 clinical trial demonstrated Adcetris plus CHP was superior to the current standard of care, CHOP, for both progression-free survival and all other key secondary endpoints, including, most importantly, overall survival. With this approval, clinicians have the opportunity to transform the way newly diagnosed CD30-expressing PTCL patients are treated,” Horwitz said.
Friedman anticipated greater use of the combination in appropriate patients at the VA.
“I expect we will be incorporating brentuximab more often as part of multiagent chemotherapy for these very rare cancers, depending on CD30 expression,” she said. “VA will likely not have a large number of patients with PTCL per year receiving this medication. However, based on the clinical trials using brentuximab, there will likely be improved disease control in patients who do receive it.”
Brentuximab had previously received approval for relapsed systemic ALCL, primary cutaneous ALCL, CD30-expressing mycosis fungoides, and for classical Hodgkin lymphoma in first-line treatment of advanced disease, as a consolidation therapy following stem cell transplantation, and after failure of SCT or failure of two or more multidrug chemotherapies in patients who are not SCT candidates.
The drug joins a growing list of agents that are transforming oncology, according to Friedman.
“The treatment of cancers, including hematologic malignancies such as PTCL is undergoing a major change. Part of that is targeted therapies, such as brentuximab, and companion diagnostics/testing that will be part of the new diagnostic and treatment landscape,” she noted.
To fully personalize treatment and optimize outcomes, clinicians still need greater understanding of the differences between cancers, even within the same overall type, Friedman added. “We will need to have more-precise classification of cancers and subgroups of cancers so that we can provide targeted specialized therapies for our patients.”
1 Richardson NC, Kasamon YL, Chen H, de Claro RA, Ye J, Blumenthal GM, Farrell AT, Pazdur R. FDA Approval Summary: Brentuximab Vedotin in First-Line Treatment of Peripheral T-Cell Lymphoma. Oncologist. 2019 Mar 26. pii: theoncologist.2019-0098. doi: 10.1634/theoncologist.2019-0098. [Epub ahead of print] PubMed PMID: 30914464.
2 Carson KR, Horwitz SM, Pinter-Brown LC, Rosen ST, Pro B, Hsi ED, Federico M, Gisselbrecht C, Schwartz M, Bellm LA, Acosta MA, Shustov AR, Advani RH, Feldman TA, Lechowicz MJ, Smith SM, Lansigan F, Tulpule A, Craig MD, Greer JP, Kahl BS, Leach JW, Morganstein N, Casulo C, Park SI, Foss FM. A prospective cohort study of patients with peripheral T-cell lymphoma in the United States. Cancer. 2017 Apr 1;123(7):1174-1183.
3 Horwitz S, O’Connor OA, Pro B, Illidge T, Fanale M, Advani R, Bartlett NL, Christensen JH, Morschhauser F, Domingo-Domenech E, Rossi G, Kim WS, Feldman T, Lennard A, Belada D, Illés Á, Tobinai K, Tsukasaki K, Yeh SP, Shustov A, Hüttmann A, Savage KJ, Yuen S, Iyer S, Zinzani PL, Hua Z, Little M, Rao S, Woolery J, Manley T, Trümper L; ECHELON-2 Study Group. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019 Jan 19;393(10168):229-240.
