
In an example of non-pharmacological treatment, Army veteran Gary Pauley, a Parkinson’s disease patient at the VA, is now a certified level 1 weightlifting coach helping others in his community – Savannah, GA — with movement disorders.
PHILADELPHIA – For five decades, physicians have used carbidopa/levodopa to treat the rigidity, tremors and slowed movement associated with Parkinson’s disease. For just as long, they’ve struggled to manage the disruptive and often painful “off episodes” that occur when the combination wears off or fails to work, but recent therapeutic advances have dramatically increased the options available to more consistently control troubling symptoms.
“There are a number of pharmacological agents that have come out over recent years considered adjunctive to carbidopa/levodopa to smooth out off-episodes,” said John E. Duda, MD, national director of the VA’s Parkinson’s Disease Research, Education and Clinical Centers; director of the Philadelphia PADRECC and co-director of the Center for Neurotrauma, Neurodegeneration and Restoration, both based at the Michael J. Crescenz VAMC in Philadelphia; and an associate professor of neurology at the Perelman School of Medicine at the University of Pennsylvania.
In Parkinson’s disease, misfolded alpha-synuclein proteins aggregate in the dopamine-producing neurons involved in the motor pathways and trigger neuronal death. As the neurons die, dopamine levels in the brain drop, and issues with movement and tremors develop.
Carbidopa/levodopa transforms into dopamine in the brain, reducing the symptoms of PD. Over time, however, response to the drug declines, and patients experience more and longer periods when the medication isn’t working and symptoms recur.
“We used to believe that starting carbidopa-levodopa later and using other agents initially might be more beneficial, but now we’re starting to use it earlier on to treat disabling and bothersome symptoms,” Duda told U.S. Medicine.
“None of the treatments available change the progression of the illness,” Duda added. “You need the neurons for levodopa to work, but patients continue to lose those neurons over time.”
Increasing the dosage or frequency can help, but high levels of carbidopa-levodopa cause dyskinesia, uncontrolled or abnormal movements.
Smoothing Delivery.
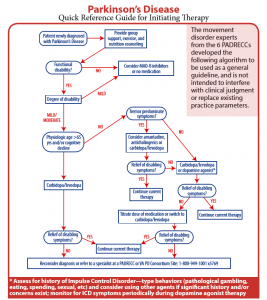 Several new formulations and delivery methods for carbidopa-levodopa minimize off episodes.
Several new formulations and delivery methods for carbidopa-levodopa minimize off episodes.
Rytary combines immediate and extended release formulations of carbidopa/levodopa in a capsule to maintain plasma concentrations of the drug for a longer period. Concentrations peak after about 60 minutes, then stay fairly level for four to five hours before dropping.
The combination received approval from the U.S. Food and Drug Administration in 2015. Several clinical trial results showed more than an hour reduction in off time per day in patients taking Rytary compared to those taking immediate release carbidopa-levodopa or immediate release caridopa-levodopa plus entacapone (Stalevo). Entacapone is another drug that reduces the breakdown of levodopa, increasing and smoothing the levels of dopamine in the brain.
“Using extended release/regular release combination is a reasonable option for off episodes,” Duda noted.
“We also use Inbrija, an orally inhaled levodopa formulation,” he added. “It just came out last year and is starting to be used to minimize off episodes when motor fluctuations are difficult to manage.”
Inbrija received FDA approval last December. It acts as the equivalent of a rescue inhaler for asthmatics but for patients with Parkinson’s. When symptoms begin to reemerge between carbidopa-levodopa doses, a patient can inhale a powdered form of levodopa that delivers a dose deep into the lungs. By bypassing the gut, the dose enters the bloodstream more quickly and at a more reliable level.
A Phase 3 clinical trial showed symptom reduction within 10 minutes and a daily reduction of 1.32 to 1.42 hours in off time. Parkinson’s symptoms improved throughout the 52-week trial. Notably, sucking action activates the inhaler, so patients with motor issues do not have to struggle to press or manipulate it while inhaling.1
An intestinal infusion pump for a gel formulation of carbidopa-levadopa (Duopa) is also available. The pump delivers 16 hours of the drugs directly to the small intestine. Duopa is specifically recommended for people with advanced PD who experience motor fluctuations. In advanced disease, emptying of the stomach becomes more erratic and slower, affecting the absorption into the bloodstream. Delivery to the small intestine avoids that issue, but requires a percutaneous gastrojejunostomy.
“Duopa frankly has a limited number of good candidates,” Duda noted. “It can be useful for certain patients, but a lot of people don’t like having a bag they have to fill every night.”
“For patients experiencing a sudden off episode, apomorphine, an injection of a dopamine agonist, can be helpful,” he added. Apomorphine has been used for 15 years in the U.S. Because the drug is associated with a very high incidence of nausea and vomiting, patients are required to pretreat with the antiemetic trimethobenzamide. Patients may also experience pain and nodules at injection sites. The FDA rejected an application for a sublingual formulation of apomorphine in January.
Safinamide is another adjunctive medication for Parkinson’s disease, designed to be taken once daily by patients on a stable dose of carbidopa-levodopa. It received FDA approval in 2017.
Nonpharmacological options
In addition to offering a full range of pharmacologic agents for treatment of PD, the VA has undertaken “a lot of research that has affected care,” Duda said.
A large study of deep brain stimulation for Parkinson’s disease with a five-year follow-up has just concluded at the San Francisco PADRECC. “The initial report will be likely be out before the end of the year. It’s given us more insight into which type of surgery is best utilized for particular outcomes,” Duda added.
Deep brain stimulation is an option for veterans who fail to respond to medication. The procedure implants a device in the chest that send electrical signals to stimulation electrodes in a targeted region(s) of the patient’s brain. DBS can significantly reduce tremors, involuntary movement and off episodes.
The procedure is typically done on awake patients, but Paul Larson, MD, PhD, chief of neurosurgery and director for the Center for Advanced Neurosurgical Operative Procedures at the San Francisco VA Health Care System, “has been developing MRI [magnetic resonance imaging]-guided DBS, in which the patient is asleep through the whole procedure,” Duda said. “It’s weird to have someone poking around in your brain when you’re awake, and we can get as good results with interoperative MRI.”
As a bonus, the DBS “studies serendipitously show huge progress in tinnitus, which is a big issue for veterans,” he added. A recent study at the VA also showed DBS confers a modest survival advantage.2
Still, “not everyone wants a hole in their head,” Duda said, and the VA has continued to explore other options.
“A new advance becoming available is focused ultrasound therapy in which a large machine shoots ultrasounds to cause a small lesion in the brain. Kind of like ablation therapy, this burns part of the brain without cutting any skin,” he explained.
“It’s been used in essential tremor and now in Parkinson’s disease as well. It’s particularly good for veterans we’re worried about who live a long way away from a center as it’s a once and done process and for those who have treatment resistant tremor on one of the body,” Duda noted.
Integrative therapy plays a role in improving PD symptoms, too. At the West Los Angeles VAMC, veterans with PD are encouraged to take yoga and tai chi classes, and the program uses acupuncture and other aspects of integrative care. The San Francisco VAMC has developed an integrative care model for end of life care.
“At the Philadelphia PADRECC, we got tired of waiting for breakthrough medications and started a brain wellness clinic that focuses on social connection, fitness, nutrition, stress reduction and sleep. That model has since been expanded to three or four other PADRECCs,” Duda said.
“While we can’t prove it slows down progression, it changes the mindset from a victim to someone fighting back against the disorder, and that’s very empowering,” he noted. “And the side effects—reduced cardiovascular risk, weight loss, improved fitness—they’re all good.”
- LeWitt PA, Hauser RA, Pahwa R, Isaacson SH, Fernandez HH, Lew M, Saint-Hilaire M, Pourcher E, Lopez-Manzanares L, Waters C, Rudzínska M, Sedkov A, Batycky R, Oh C; SPAN-PD Study Investigators. Safety and efficacy of CVT-301 (levodopa inhalation powder) on motor function during off periods in patients with Parkinson’s disease: a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Neurol. 2019 Feb;18(2):145-154.
- Weaver FM, Stroupe KT, Smith B, Gonzalez B, Huo Z, Cao L, Ippolito D, Follett KA. Survival in patients with Parkinson’s disease after deep brain stimulation or medical management. Mov Disord. 2017 Dec;32(12):1756-1763.

