HOUSTON — Mortality rates from most cancers have declined in recent years, but deaths from hepatocellular carcinoma continue to climb. Both the VA and the DoD are moving aggressively to detect and treat liver disease before it progresses to hepatocellular carcinoma (HCC) and to quickly identify patients who have developed this devastating disease.
HCC accounts for about 85% of all liver cancers. Several circumstances make this cancer more common among veterans and possibly servicemembers than the general population. First, it affects two to three times as many men as women. Second, its primary risk factors—viral hepatitis B or C, alcohol use, smoking, diabetes, metabolic syndrome, and obesity—occur at higher rates in those who have worn the uniform of U.S. forces.
“While lung cancer numbers are relatively stable, HCC numbers are skyrocketing. They’re going to keep going up because of fatty liver disease,” said David Ross, MD, PhD, director of the VA’s HIV, Hepatitis, and Related Conditions Programs. Fatty liver disease can arise in the context of alcohol use disorder as well as in connection with diabetes, metabolic syndrome, and obesity.
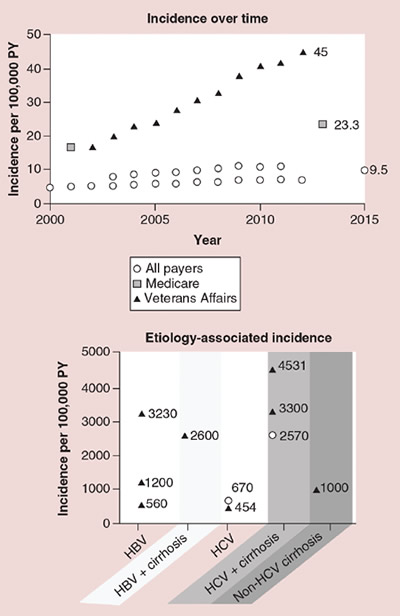
Each symbol represents an estimate from a given study. HBV: Hepatitis B virus; HCV: Hepatitis C virus; PY: Person-years. (Incidence over time) All payers sources: Ha, 2016 (SEER) [24]; Rich, 2019 (SEER) [19]; and White, 2017 (US Cancer Statistics registry) [23]; Medicare source: Shiels, 2019 [21]; Veterans Affairs source: Beste, 2015 [16]. (Etiology-associated incidence) Choi, 2015 [25]; El-Serag, 2016 [26]; Ioannou, 2018 [27]; Li, 2018 [28]; Mittal, 2014 [29]; Park, 2019 [30]; Su, 2018 [31].
Source: Epidemiologic, humanistic and economic burden of hepatocellular carcinoma in the USA: a systematic literature review; Hepatic Oncololgy; www.futuremedicine.com/doi/10.2217/hep-2020-0024
A national study found that up to half of HCC patients in the United States receive no treatment for the cancer, with the likelihood of non-treatment highest among older patients, African Americans, the uninsured, and those covered by Medicaid. Median survival for untreated patients is 13.4 months for those in whom the cancer is detected in early stages, 9.5 months for intermediate stage patients, and just 3.4 months for those with advanced cancer, and 1.6 months for patients with end-stage disease.1
“The high mortality rate attributed to HCC is largely driven by late stage diagnoses, for which treatment options are limited and median survival is typically 1-2 years,” according to a study by VA researchers at the Michael E. DeBakey VAMC and the Baylor College of Medicine, both in Houston, and the University of Texas Southwestern Medical Center in Dallas, Texas.2 “Given the association between early tumor detection and improved survival, the [American Association for the Study of Liver Disease and the European Society for Liver Disease] recommend semi-annual HCC surveillance using abdominal ultrasound with or without alpha fetoprotein (AFP) for at-risk populations, including those with cirrhosis.”
Patient-related Barriers
To better understand what drove late stage diagnoses, this group of researchers examined barriers to surveillance for HCC in patients with cirrhosis, which sharply increases the risk of HCC.
Their study included 1,020 patients with cirrhosis who received care through Parkland Health System, a safety net health system (61.7%); the Houston VAMC (23.1%); or Southwestern Medical Center (15.2%). While more than 80% of patients knew that cirrhosis increased their risk of HCC, that ultrasound is used for surveillance, and surveillance increases detection of early stage HCC, 25% did not know that early stage HCC was curable and more than 40% did not realize surveillance was needed even if their physical exam and laboratory results were normal.
Notably, while nearly 60% reported that receiving HCC surveillance reduced their concerns about dying of HCC, only one-third of the patients received the recommended semi-annual surveillance.
Still, more than half of the patients(51.2%) said that receiving surveillance posed significant challenges. The cost of surveillance tests was an obstacle for 28.9% and 42.8% were generally concerned about affording healthcare. Veterans were half as likely to worry about paying medical bills compared to the other patients.
Other difficulties included scheduling ultrasound exams, arranging transportation for testing, and uncertainty about where to go for surveillance. In addition, 17.8% said they weren’t certain about the need for surveillance even though their provider recommended it, nearly 10% considered the length of time needed for the ultrasound off-putting, and 13% failed to schedule surveillance because they feared a cancer diagnosis.
Provider-related Barriers
Not all of the delays in screening and surveillance arise on the patient side, however, as another group of VA researchers from the Michael E. DeBakey VAMC determined.
In a study published in Clinical Gastroenterology and Hepatology in July, they found that almost half of 655 veterans with cirrhosis who were diagnosed with HCC between 2006 and 2011 experienced a delay of more than 60 days from the time of a “red flag” for HCC and actual diagnosis. Using the American Association for the Study of Liver Disease (AASLD) guidelines as of 2005, red flags included liver lesions of less than 1 cm in diameter (requiring follow up every three to four months), liver lesion of 1-2 cm diameter (needing follow up or classification as HCC), and lesions larger than 2 cm with characteristic arterial vascularization of alpha fetoprotein greater than 200 ng/mL (meeting the definition of HCC).
Among patients who had delays of more than 60 days, the median time from initial red flag to HCC was 6.8 months (95% CI 5.9-7.9 months).
Patient factors such as missing or cancelled appointments accounted for some delays, but “in a third of patients, providers did not adhere to AASLD guidelines,” the authors wrote. “This may be in part due to confusion among providers about which practice guideline to follow, as well as the complexity of HCC diagnostic imaging criteria and radiology reporting.”
Lack of provider adherence to the guidelines were significantly associated with delays of more than two months (adjusted odds ratio [OR], 4.82; 95% CI, 3.12–7.45). In addition, a diagnostic imaging evaluation instead of measurement of alfa fetoprotein measurement increased the odds of delays (adjusted OR, 2.63; 95% CI, 1.09–6.24) as did making the diagnosis incidentally during examination for an unrelated medical problem rather than during an HCC-related assessment (adjusted OR, 2.26; 95% CI, 1.09–4.67).
The researchers also identified issues with initial diagnostic assessment, diagnostic test performance and interpretation, and diagnostic follow-up and coordination as reasons for delayed diagnosis.
To address the delays, the authors recommended “standardized educational training for physicians, establishing multi-disciplinary clinics for patients at high-risk patients for HCC, and increased utilization of HCC tumor boards in the diagnostic process.”
They also advised system alerts for abnormal liver imaging, including guideline recommendations for additional diagnostics in radiology reports, and standardized radiology reporting templates for Li-RADS criteria. Li-RADS is a system used by the VA which has standardized imaging diagnostic criteria, but its complexity may require interpretation by a radiologist with liver imaging experience, which many smaller centers lack.
- Aly A, Ronnebaum S, Patel D, Doleh Y, Benavente F. Epidemiologic, humanistic and economic burden of hepatocellular carcinoma in the USA: a systematic literature review. Hep Onc. July 2020;7(3).
- Choi DT, Davila JA, Sansgiry S, David E, Singh H, El-Serag HB, Sada Y H-F. Factors Associated with Delay of Diagnosis of Hepatocellular Carcinoma in Patients with Cirrhosis. Clin gastroenterol Hepatol. 2020 Jul 18;21542-3565(20)30988-5 doi: 10.1016/j.cgh.2020.07.026. Online ahead of print.
