HOUSTON—The VA has announced that it expects to eliminate hepatitis C in all veterans willing and able to receive treatment by May of this year.
Successful treatment for HCV also dramatically reduces the risk of hepatocellular carcinoma and other complications. The VA reported last month that “the overall death rate one year after treatment reduced to 80% among veterans in VA care with HCV. Veterans cured of HCV with [direct-acting antivirals] were also 84% less likely to develop liver cancer.”
Direct-acting antivirals first came on the market in 2014, enabling all-oral treatment of HCV in eight to 12 weeks with few adverse effects and achieved sustained virological response or cure in 94% to 95% of patients. Those drugs represented a huge step forward compared to previous therapies, which required weekly injections of interferon, with its significant physical and psychiatric side effects, and had cure rates of 35%-55%, depending on the therapy and genotype of hepatitis C.
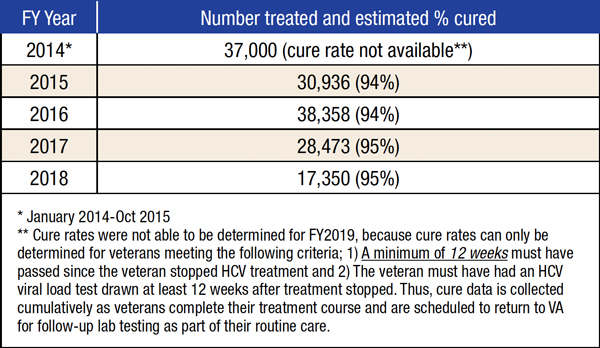
Starting in early 2014, the VA aggressively pursued the goal of eliminating HCV among all veterans in care, at one point starting treatment for a new patient an average of nearly every minute of every work day. So far, 116,000 veterans have been treated with direct-acting antivirals, and 96,654 have been cured. Fewer than 27,000 veterans who receive care through the VHA remain to be treated.
“This is an incredible public health success story,” pointed out Fasiha Kanwal, MD, MSHS, an investigator in the Clinical Epidemiology & Comparative Effectiveness Program at the Center for Innovations in Quality, Effectiveness and Safety at the Michael E. DeBakey VAMC, chief of the Gastroenterology and Hepatology Section at the Baylor College of Medicine, both in Houston, and editor-in-chief of Clinical Gastroenterology and Hepatology.
“Within the VA, we have documented that the risk of hepatocellular carcinoma was increasing 10-fold among patients with HCV until very recently. Direct-acting antivirals are very, very effective and safe and have had a huge impact on HCC as well as HCV,” Kanwal told U.S. Medicine.
Risk Reduction
“Patients who achieve a sustained virological response with direct-acting antivirals have a 60% to 75% reduction in HCC risk,” Kanwal said. “Every study has found a similar, remarkable reduction in risk of HCC. That’s a major difference in a disease in which nearly everyone who has it dies.”
That reduction in risk should not lead to complacency, however. “Treatment reduces risk, but it doesn’t drop to zero,” Kanwal noted. Two groups continue to have a significantly elevated risk of HCC even after treatment.
“In patients who had already developed advanced fibrosis or cirrhosis before eradicating chronic hepatitis C, a residual risk of these complications unfortunately persists even after viral eradication. This is particularly true of the risk of hepatocellular carcinoma,” explained Akbar K. Waljee, MD, director of the Inflammatory Bowel Disease Program at the VA Ann Arbor Healthcare System, associate director of the Michigan Integrated Center for Health Analytics and Medical Prediction and associate professor in internal medicine at the University of Michigan.
Between 30% and 40% of veterans treated for HCV had cirrhosis at the time of treatment. “Risk remains high in this group,” said Kanwal, “and clinicians should consider liver cancer surveillance.”
A VA study quantified just how much higher the risk for this group is. Led by George Ioannou, MD, MS, of the VA Puget Sound Healthcare System and professor of gastroenterology at the University of Washington in Seattle, the study found that patients who had cirrhosis prior to successful treatment had an HCC incidence rate eight times that of patients who did not have cirrhosis, 1.97 per 100 patient-years vs. 0.24 per 100 patient-years. 1
In keeping with these findings, American Association for the Study of Liver Disease guidelines note that “the risk of HCC for patients with HCV-related cirrhosis who develop SVR after DAA treatment is lowered, but not eliminated, and therefore patients with cirrhosis and treated HCV should continue to undergo surveillance.”
Patients who do not achieve sustained virological response following treatment for HCV constitute the second group that continues to have a higher risk of HCC. A VA study led by Kanwal determined that achieving SVR reduced the risk of HCC by 68% in patients with cirrhosis and 82% in those without cirrhosis compared to patients who did not achieve SVR. 2
Achieving SVR
Ongoing surveillance is only recommended for patients who fail to achieve SVR and also have cirrhosis.
“The beneficial effect comes from attaining SVR,” Kanwal reiterated. But patients who previously failed treatment with an interferon-based therapy or one of the direct-acting antivirals do necessarily have to resign themselves to a higher risk forever.
“More than 95% now achieve cure within one round, but patients can repeat treatment if the first course fails,” she said.
The opportunity to receive treatment before developing complications, let alone twice, distinguishes HCV care at the VA.
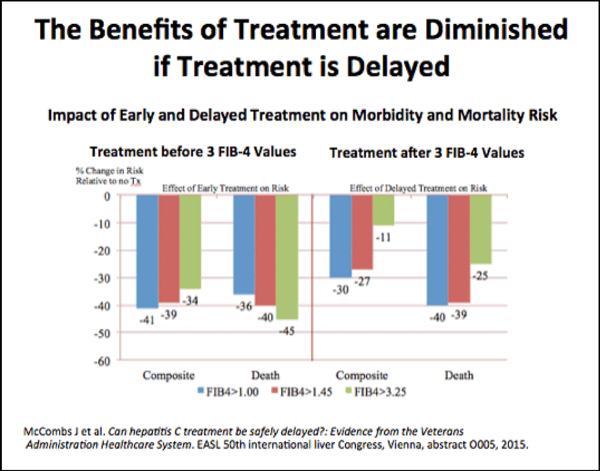
“We should try to treat patients before they progress to cirrhosis,” Kanwal said. “That’s not an issue in the VA, but outside of the VA, treatment is often restricted to those who have cirrhosis. That misses the point; those patients remain at risk for HCC. You don’t have to worry about HCC progression if you treat HCV early.”
1. Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017 Sep 5. pii: S0168-8278(17)32273-0.
2 Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology. 2017 Oct;153(4):996-1005.e1.
