New Treatments Provide Reasons to Measure Progress
ELK GROVE VILLAGE, IL – With new drugs approved for treatment of spinal muscular atrophy, patients and caregivers of patients now have reason to measure progress and to assess quality of life.
Historically, spinal muscular atrophy (SMA), a genetic neuromuscular disease characterized by progressive muscle atrophy and weakness, often led to paralysis and premature death. SMA usually is first diagnosed in infants and toddlers, but also can be initially identified in children, and less commonly, adults.
The online 2019 Cure SMA Community Update Survey, with results published recently in the Orphanet Journal of Rare Diseases, assessed health-related quality of life (HRQoL), loss of work productivity, and fatigue using the Health Utilities Index Questionnaire (HUI), the Work Productivity and Activity Impairment Questionnaire (WPAI), and the Patient Reported Outcomes Measurement Information System Fatigue Short Form (PROMIS Fatigue SF), respectively, among SMA patients and caregivers.1
The goal was to collect baseline quality of life results using the above Patient Reported Outcome Measures (PROMs).
“The 2019 Community Update Survey dataset provides an important benchmark from which to begin assessing year-over-year change in HRQoL for affected individuals and their caregivers,” the authors explained. “Cure SMA will conduct follow up annual surveys using the WPAI and HUI instruments to evaluate the impact that new therapies are making on the overall experience of affected individuals and their families. It is anticipated that these future survey activities will also add in other HRQoL measurements (including the EQ-5D and the Fatigue Impact Scale to broaden the picture of SMA impact among the community, evaluate which tools are most sensitive to each subtype of the diverse SMA population, can assess treatment affects and determine the health utility among a large sample of affected individuals with SMA.”
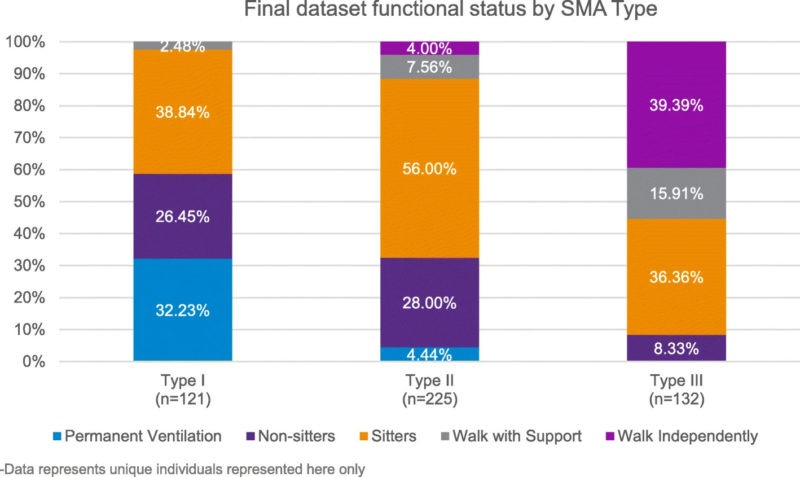
Researchers received 666 surveys completed between March and May 2019, and included 478 in the analysis, taking into account duplicates, missing data, or deaths. Most of the responses involved SMA type II, 47%, which is less severe than type I: with 25% for type I, the most severe, and 28% for type III, which is considered milder.
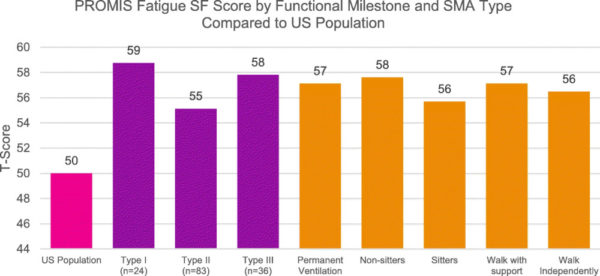
Current functional status/milestones were used to categorize the responses, with subsets for “permanent ventilation,” “non-sitters,” “sitters,” “walk with support,” and “walk alone.” While WPAI and HUI respondents included affected adults and caregivers, the PROMIS Fatigue SF was completed by the primary caregiver of affected children.
Results indicated a highly rated burden of SMA across all three assessments; that was in line with findings from previous qualitative studies that assessed the burden of SMA across phenotypes. As measured by the HUI, the quality of life scores fell under the category of “severe disability:” work productivity lost due to having SMA or caring for someone with SMA was also significant; and the fatigue levels of children affected with SMA was greater than that of the general population regardless of type.
“Overall, those affected by a less severe form of SMA and with a higher functional status reported higher HRQoL and lower work productivity and activity impairment,” the authors reported. “All affected individuals reported higher fatigue levels than the general population.”
Baseline Snapshot
The authors noted that the study offered essential insights into the burden of SMA among affected individuals and their caregivers. A key advantage is that the results provide a baseline picture of the patient and caregiver experience with SMA in a post-treatment era from which to measure year-over-year changes in quality of life scores from new therapies and improved care,” they pointed out.
Some of the measurements were problematic, however. “The WPAI demonstrates the significant impact of work productivity among SMA populations,” the researchers explained. “Aspects of the HUI seem more appropriate to certain SMA sub-populations than others. Measures from the PROMIS Fatigue SF appear to under-represent the burden of fatigue often reported by SMA individuals and caregivers; this may, perhaps be due to a lack of sensitivity in the questions associated with fatigue in the SMA affected population, when compared with other studies on this topic. Overall, these results suggest the need for SMA-specific quality of life outcome measures to fully capture clinically meaningful change in the SMA population.”
The bottom line, according to the study, is the immense challenges faced by patients and their families. “Often this odyssey begins with a prolonged and traumatic process to confirm diagnosis and a life-long journey of overwhelming physical, emotional, psychosocial, and financial strains associated with managing and living with a progressive, debilitating, and incurable disease.”
The study described the situation as “critical” when it comes to quantifying outcomes that are meaningful from the patient perspective and how those are affected by new therapies.
Researchers also advised that both patients and healthcare providers do not believe that factors being assessed in pharmacoeconomic analyses are not sensitive enough to capture the overall effects on quality of life. impact on quality of life. “Thus, it has been proposed for SMA (and for rare disease more generally) that focusing on generating evidence that translates therapy benefit from clinical trials to patient and family relevant outcomes such as quality of life, independence and productivity impact might be more appropriate when analyzing cost effectiveness,” according to the authors. “Encompassing these additional dimensions would provide a more complete picture of the burden of disease and the potential overall impact of a new therapy.”
They noted that similar efforts have recently been undertaken in other rare, pediatric diseases such as Duchenne Muscular Dystrophy (DMD).
The authors concluded that the 2019 Community Update Survey dataset provides an important benchmark from which to begin assessing year-over-year change in HRQoL for affected individuals and their caregivers. Cure SMA said it will conduct follow up annual surveys using the WPAI and HUI instruments to evaluate how new therapies are affecting the overall experience of affected individuals and their families.
“Ultimately, we anticipate learning through this process, that different instruments will be more appropriate for assessing HRQoL within SMA, and among the various SMA subpopulations, ages of affected individuals and functional milestone status,” the researchers wrote.
- Belter L, Cruz R, Jarecki J. Quality of life data for individuals affected by spinal muscular atrophy: a baseline dataset from the Cure SMA Community Update Survey. Orphanet J Rare Dis. 2020 Aug 24;15(1):217. doi: 10.1186/s13023-020-01498-2. PMID: 32838797; PMCID: PMC7447573.