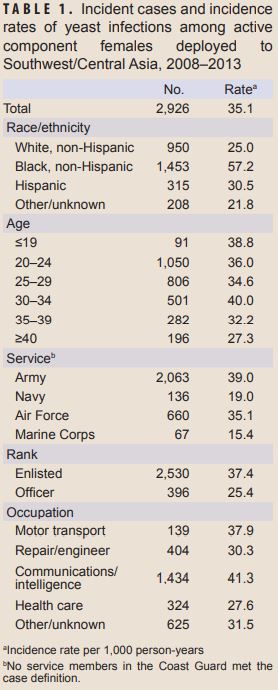
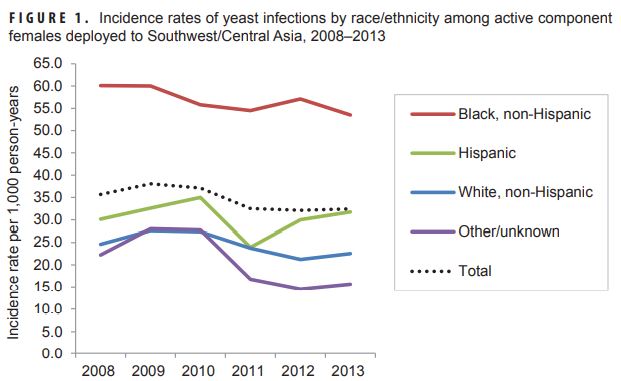
Combat Zones, Field Exercises Increase Risks for Military Women
BIRMINGHAM, AL — Candida, a common yeast, frequently cohabitates on skin and inside the body and typically causes little trouble. When conditions enable it to multiply rapidly or when drug-resistant strains take hold, however, it can cause a range of problems, including vulvovaginal candidiasis, one of the most common infections in women.
An estimated 75% of women will experience a vulvovaginal yeast infection at some point in their lives. Pregnancy, hormonal contraceptives, diabetes, immunocompromise, and antibiotic treatment all increase the risk of vulvovaginal candidiasis (VVC). For military women, the risk increases in combat zones and during field exercises.
Symptoms can be range from annoying itching to acutely painful urination or intercourse. Clinicians often prescribe an antifungal treatment over the phone and over-the-counter therapies are also available and serve as first-line treatment for many women, particularly those who have previously experienced a vaginal yeast infection. It is important to note that up to 20% of women normally have vaginal Candida with no symptoms and do not require treatment.
Standard Treatment
Typically, VVC is treated with regimens requiring one to three days of therapy with fluconazole or other azoles. Over-the-counter intravaginal preparations of clotrimazole, miconazole and tioconazole are the recommended first-line therapies for simple vulvovaginal candidiasis. Prescription therapies include topical butoconazole or terconazole or oral fluconazole. Fluconazole is contraindicated in pregnancy.
Older treatments with lower cure rates and longer regimens include topical gentian violet, boric acid, and nystatin suppositories and flucytosine cream. Echinocandins (caspofungin, micafungin, ) and amphotericin B deoxycholate, a polyene, are other treatment options. Echnocandins are delivered intravenously. Amphotericin B is delivered by infusion and is used only for serious, life-threatening infections because of its potentially serious adverse effects. Those include anaphylaxis, loss of potassium and magnesium, cardiotoxicity, cellular toxicity and renal toxicity.
Resistance
Over the past several decades, an increasing number of Candida species have developed resistance to the most frequently used antifungals. Historically, Candida albicans has been the primary cause of vulvovaginal yeast infections, but Candida glabrata, C. tropicalis and other species account for a rising proportion of cases. In 1988, they accounted for 10% of yeast vaginitis occurrences; by 1995, the rate was 17.2% in the U.S. More recent studies have found rates as high as 45%.
The non-C. albicans species have historically shown greater resistance to antifungals, but C. albicans appears to be following their lead. In a study published this summer, three Candida species were detected in VVC samples and “resistance to at least one antifungal was detected in up to 65% of the isolates, and resistance to both antifungals [two azoles] reached a frequency of 25%,” the authors noted. “Moreover, azole-resistant isolates were distributed among all species identified.”1
More alarming, a highly resistant species, C. auris, has emerged as a cause of vulvovaginitis. “C. auris is increasingly being reported from a variety of clinical samples as a cause of candidiasis with varying degrees of severity. It is better known as a causative agent of candidaemia but has also been reported as a cause of VVC,” according to the U.S. Centers for Disease Control. Ninety percent of C. auris tested in the U.S. demonstrate resistance to fluconazole and up to 33% are resistant to amphotericin B. While most C. auris sample respond to echinocandins, they can develop resistance to this class of antifungal agents during treatment.
“Resistance to another class of antifungal drugs, echinocandins, is particularly concerning. Echinocandin resistance appears to be increasing, especially in the species Candida Glabrata. C. glabrata already has high levels of resistance to the antifungal fluconazole,” according to the U.S. Centers for Disease Control and Prevention. “Echinocandins are the preferred treatment for C. glabrata, and echinocandin resistance could severely limit treatment options for patients with candidiasis caused by C. glabrata.”
New Option Approved
In June, the FDA approved the first new treatment for vaginal yeast infections in two decades and the first in a new class of antifungal, triterpenoids, which act by interfering with biosynthesis of a key constituent of the fungal cell wall. Ibrexafungerp is the only oral therapy besides fluconazole available for VVC. Ibrexafungerp showed broad activity against Candida species, including C. auris.
The FDA based its decision on results from two phase 3 studies which showed that 63.3% of patients achieved a cure within 10 days of treatment initiation and 73.9% by day 25. Treatment was 300 mg twice in one day, taken 12 hours apart.
A phase 2 study of Ibrexafungerp conducted by Jane Schwebke, of the Birmingham VAMC and the University of Alabama, and others, found that at day 25 70.4% of women treated with Ibrexafungerp showed no signs or symptoms of VVC compared to 50% of those treated with fluconazole. In addition, patients in the Ibrexafungerp group required less antifungal rescue medication, 3.7%, vs. 29.2% for the fluconazole group.
- Rolo J, Faria-Gonçalves P, Barata T, Oliveira AS, Gaspar C, Ferreira SS, Palmeira-de-Oliveira R, Martinez-de-Oliveira J, Costa-de-Oliveira S, Palmeira-de-Oliveira A. Species Distribution and Antifungal Susceptibility Profiles of Isolates from Women with Nonrecurrent and Recurrent Vulvovaginal Candidiasis. Microb Drug Resist. 2021 Aug;27(8):1087-1095. doi: 10.1089/mdr.2020.0139. Epub 2021 Feb 26. PMID: 33646045.