Operation Warp Speed Counts on Collaborations to Combat COVID-19
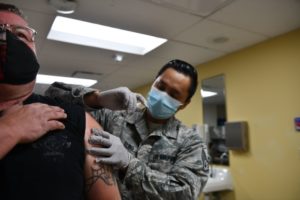
Staff Sgt. Michael Elbo, 509th Healthcare Operations Squadron NCO in charge of immunizations, administers a COVID-19 vaccine at Whiteman Air Force Base, MO, on New Year’s Eve 2020. Operation Warp Speed’s partnerships with industry helped make vaccines available as soon as possible. Air Force photo by Tech. Sgt. Alexander W. Riedel
WASHINGTON — In a year of grim news, an extraordinary public-private partnership cheered an anxious nation—and the world—with the record-breaking development of multiple vaccines for a deadly virus first detected barely 12 months earlier.
Government and industry joined forces to apply technological innovations such as artificial intelligence to sequencing SARS-CoV-2, conducting virtual testing of thousands of strategies and combing through myriad approved and developmental molecules to find those most likely to work, in the process reducing what previously took years to mere days.
Fifty years of research in academia, industry and government laboratories on the potential use of messenger RNA (mRNA) vaccines enabled Pfizer/BioNTech and Moderna/NIH to produce and gain approval for their vaccines in less than 11 months.
“On Jan. 7, before China had reported even one death from the novel coronavirus or confirmed human-to-human transmission, NIH scientists and innovators at Moderna agreed to begin work on the vaccine that received FDA authorization today,” said Department of Health and Human Services Secretary Alex Azar. “In March, BARDA scientists joined the partnership and worked with Moderna and NIH to reach commercialization, building on support BARDA has provided since 2016 for the remarkable mRNA technology.”
Operation Warp Speed, the U.S. government’s joint effort of the Department of Human Services (HHS) and the DoD, invested in industry partnerships to bring vaccines to fruition as quickly as possible. Moderna, Johnson & Johnson, AstraZeneca-Oxford, Novavax and Sanofi-GlaxoSmithKline received a total of approximately $6.5 billion to support development through clinical trials, expansion of manufacturing facilities and production of millions of doses of their vaccine candidates even as clinical trials continued. Pfizer-BioNTech received $1.95 billion to finance production and secure the right to purchase 100 million doses of its vaccine, pending U.S. Food and Drug Administration emergency use authorization.

As pharmaceutical companies came closer to an approved vaccine in November, Operation Warp Speed Chief Operating Officer Army Gen. Gus Perna, right, led a Table Top Exercise to explore COVID-19 vaccine distribution scenarios and contingency plans as pharmaceutical companies come closer to an approved vaccine. Defense Public Affairs photo by Kimberly Hanson.
By mid-December, the Pfizer-BioNTech vaccine had received EUA, followed a week later by Moderna’s vaccine. Clinical trials for both vaccines reported outstanding efficacy, both at about 95%.
“It is nothing short of a medical miracle to have FDA authorization of a vaccine for COVID-19 just over 11 months since the virus was made known to the world,” said Azar. “The triumph of Operation Warp Speed is a tribute to dedicated public servants across HHS and the Department of Defense, our partners in the private sector and incredible American scientists.”
Army Gen. Gustave Perna, MD, Operation Warp Speed’s chief operating officer, anticipated that 20 million doses of vaccine from the two manufacturers would be delivered by the end of 2020. A total of 300 million doses—enough for 150 million people—have been purchased from the two companies for delivery by July.
Johnson & Johnson could announce trial results in early January and may have sufficient data to file for an EUA for its single-shot vaccine later that month. The AstraZeneca/Oxford University vaccine could complete U.S. clinical trials and file for authorization in late January or February. Novavax also expects wrap up its trials and submit its application to the FDA in the first quarter of 2021. Sanofi-GlaxoSmithKline’s vaccine will likely be ready in the second half of the year.
Throughout the vaccine development and distribution process, “We’ve seen unprecedented levels of collaboration between the government and industry on behalf of the American people,” said Army Brig. Gen. Michael “Mac” McCurry, Operation Warp Speed’s deputy for security and assurance.
Beyond Vaccines
At the end of December, Operation Warp Speed agreed to support the advanced development and large-scale manufacturing of Merck’s investigational therapeutic MK-7110 for the treatment of hospitalized patients with severe or critical COVID-19. MK-7110 represents a first-in-class fusion protein and immune modulator that the company hopes can rein in an excessive immune response cascade, limiting the damage to organs.
The agreement provided $356 million to Merck to take the potential therapeutic through clinical trials, submission to the FDA and delivery of 100,000 doses by June 30, 2021, pending granting of emergency use authorization. As with the vaccines, the upfront money will enable Merck to ramp up production of MK-7110, prior to the FDA’s decision.
Based on early results of a Phase 3 clinical trial, MK-7110 has a good chance of receiving authorization—it increased the chance of clinical recovery 60% compared to placebo and cut the risk of respiratory failure or death by 50% compared to current standard of care.
“Our agreement with Merck is the latest example of how industry and government are coming together under Operation Warp Speed to move potential therapeutics all the way from development through to manufacturing, enabling faster distribution,” Azar said.
At the end of the year, the U.S. Army announced another pandemic-related collaboration. The U.S. Army Medical Research and Development Command (USAMRDC) funded a clinical trial of the BioIntelliSense BioSticker device for early detection of COVID-19 symptoms combined with Philips’ remote patient monitoring platform.
“Key industry and academic partnerships provide DoD a timely opportunity to field medical-grade wearables capable of high-frequency physiologic surveillance,” stated Commander Christopher Steele, director of the Military Operational Medicine Research Program at USAMRDC. “Our goals are to capitalize on mature, wearable tech and validate predictive algorithms to identify COVID-19 positive individuals that have yet to show clear medical symptoms. Outputs can directly maximize military preparedness and provide immediate benefit for the general population as these tools can be used outside of medical treatment facilities.”
