In a 1963 photo, a U.S. Army Huey helicopter sprayed Agent Orange over agricultural land during the Vietnam War. Veterans who develop CLL and other chronic B-cell leukemias and were exposed to Agent Orange do not have to prove a connection between their disease and service to be eligible to receive VA health care and disability compensation. Source: National Archives
SAN ANTONIO — As with most cancer, overall survival in chronic lymphocytic leukemia has steadily risen over the past two decades. New agents play an important role in that improvement, but they also require additional training and close attention to different types of adverse effects.
Managing each patient’s treatment requires an awareness of the likely risks and potential benefits of a growing number of therapeutic options. “The treatment landscape has become very complex, and oncologists need to be aware of all the choices and bring them into practice sooner rather than later, but it’s a big challenge to stay updated,” Zohra Nooruddin, MD, a staff hematologist at the South Texas Veterans Health Care System in San Antonio, told U.S. Medicine.
It also necessitates a keen understanding of the differences between participants in a clinical trial and the patient being seen in a clinic, who is typically older and has more comorbidities. As the result of a constellation of factors, many of those older, sicker patients happen to be veterans. CLL accounts for almost 40% of all adult leukemias and affects 20,000 people in the U.S. each year. Exposure to Agent Orange and other herbicides during the Vietnam War or in the Korean Demilitarized Zone, as well as to contaminated water at Camp LeJeune, increases the risk of later development of CLL. As a result, the blood disorder is covered as a presumptive disease by the VA.
Two demographic factors also raise the risk of CLL, and both are found at a high rate among veterans receiving care through the VA. The disease disproportionately hits the elderly, with an average age of 70 at diagnosis, and occurs at higher frequency in men than women. CLL also is more likely to kill older men than other patients, according to a study presented at the 2021 virtual American Society for Clinical Oncology conference in June.1
The retrospective study looked at estimated survival rates before and after the introduction of targeted therapies for patients with CLL using records from the Surveillance, Epidemiology and End Results (SEER) database. The researchers found that the overall five-year relative survival rate rose from 73.7% for patients diagnosed between 1985 and 1989 to 89.4% in those first diagnosed between 2010 and 2014. Breaking the numbers down further reveals that for male patients the five-year survival increased from 51.6% to 75% during that time period, while for female patients it climbed from 76.8% to 90.8%.
The prevalence and impact of CLL on veterans has made research on the disease a matter of significant interest for the VA. At the same time, the department has focused on understanding the impact of new and more effective agents on both outcomes and costs as new guidelines incorporate the latest developments.
NCCN Recommendations
Despite the number of Food and Drug Administration-approved therapies, the latest National Comprehensive Cancer Network guidelines for CLL/SLL (4.2021) lists the same three therapies as the preferred first-line regimens for patients with and without del(17p)/TP53 mutation as well as for frail patients who have significant comorbidities, patients over age 65, and those younger than 65 without significant comorbidities—in other words, for virtually all patients. They are acalabrutinib with or without obinutuzumab, ibrutinib and venetoclax with obinutuzumab. SLL is the same cancer as CLL, but it affects the lymph nodes or spleen instead of the blood and bone marrow.
All three are also preferred second-line and subsequent therapies, along with duvelisib and idelalisib plus rituximab, for all categories of patients. Venetoclax is an additional preferred second-line therapy for patients with del(17p) and TP53 mutation. Notably, chemotherapy and chemoimmunotherapy (CIT) are no longer preferred, although the NCCN guidelines include them as other recommended regimens.
Ibrutinib vs. CIT
While the guidelines are clear about the limited role for chemotherapy and CT, researchers sought to better understand the real-world impact of the switch to novel agents in veterans with CLL and SLL in terms of outcomes, health care resource utilization and costs. They used the VHA database to identify veterans diagnosed with CLL between April 1, 2013, and March 31, 2018. To be included, patients also needed to have a prescription claim for ibrutinib or any combination of NCCN-recommended CIT for CLL. The researchers excluded veterans who received ibrutinib in combination with a CD20 agent.2
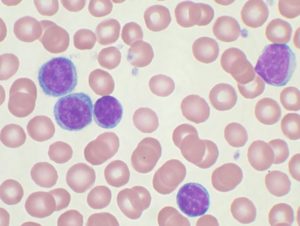
High-power magnification (1000 X) of a Wright’s stained peripheral blood smear showing chronic lymphocytic leukemia (CLL). The lymphocytes with the darkly staining nuclei and scant cytoplasm are the CLL cells.
During the period studied, ibrutinib was the only novel agent listed as a preferred regimen in the NCCN guidelines. Acalabrutinib and obinutuzumab gained FDA approval for CLL in 2019. The FDA granted approval to venetoclax in the CLL setting in 2016 but only for relapsed/refractory CLL in patients with the del(17p) alteration. It received an expanded indication in mid-2018 as a finite therapy in combination with rituximab.
The study included 7,787 patients who received ibrutinib in the first line and 1,039 who received CIT as their first treatment. Following propensity score-matching, 614 patients received first-line ibrutinib and first-line CIT, while 149 were in the first- and second-line ibrutinib cohorts. Among the first line CIT patients, 57.5% received bendamustine plus rituximab (BR), 14.4% received fludarabine, cyclophosphamide and rituximab (FCR), and 9.0% started with chlorambucil plus obinutuzumab.
Among the patients who received first-line ibrutinib, 18.6% received a second-line therapy. For 63% the second-line treatment was also ibrutinib, following a break of at least 90 days. Just under 40% of CIT patients received a subsequent line of therapy, with 45.2% of them receiving ibrutinib in the second or third line.
The researchers determined that those who received ibrutinib first had a significantly longer time to next treatment than either those who received CIT in the first line or those who received ibrutinib in the second line. The first-line ibrutinib group was half as likely to start another line of treatment as the first-line CIT group and was about 60% less likely to start a new treatment than the second-line ibrutinib group. “These findings demonstrate that among U.S. veterans with CLL/SLL, 1L ibrutinib use was associated with significantly longer TTNT versus that of 1L CIT,” the authors said. “Similarly, early treatment with ibrutinib was associated with longer TTNT as compared to ibrutinib use in later lines of therapy.”
Patients in the first-line ibrutinib group also had fewer inpatient visits (rate ratio [RR] = 0.38; 95% CI = 0.28-0.52; P ≤ 0.05) and outpatient visits per person per month (RR = 0.72; 95% CI = 0.68-0.77; P ≤ 0.5). Their medical costs were also more than $7,000 lower per month. The team concluded that “[l]ower health care resource utilization and medical costs with [first-line] ibrutinib offset higher pharmacy costs incurred by U.S. veterans.”
Discontinued Therapy
With a growing number of veterans with CLL starting treatment with novel therapies, VA researchers in San Antonio sought to determine what factors led to discontinuation of those therapies. They reviewed the records of 1,205 CLL patients who began treatment with a novel oral agent at the VA between October 1, 2013, and March 31, 2018.
About one-third of veterans receiving ibrutinib in the first or second line discontinued therapy, with two-thirds stopping the treatment because of adverse events. “The majority of VHA patients on a novel CLL agent in this study received ibrutinib monotherapy in an [relapsed/refractory] setting,” the authors wrote. “This is not unexpected as ibrutinib did not receive FDA approval for the [first-line] setting until 2016.”
For venetoclax in relapsed/refractory CLL, 31% discontinued therapy, and 41% of those did so because of adverse events. Idelalisib had a much higher rate of discontinuation, 84%, more than half of which was driven by adverse events. In keeping with the finding of this study, the authors noted that, “[a]lthough the idelalisib-rituximab regimen was approved in 2014, its use is limited in the VHA and mainly in the R/R setting due to its safety profile.”
Overall, “we identified higher rates of discontinuation than documented in the clinical trial settings,” they said. “The rate of discontinuation of 1L ibrutinib in our study was 33%, compared with the 21% rate reported in RESONATE-2 at similar follow-up time.” The team found similar results for subsequent lines of ibrutinib and attributed the higher rates to the older population with more comorbidities served by the VA compared to participants in the clinical trials.
“This real-world study suggests that AEs play an important role in dose reductions and discontinuations;” the researchers noted, “however, physician inexperience in using these drugs when they were first introduced could be part of what is leading to these negative outcomes.”
- Alrawashdh N, McBride A, Persky D, Sweasy J, Erstad B, Abraham I. Survival trends in chronic lymphocytic leukemia in the era of oral targeted therapies in the United States: SEER database analyses (1985 to 2017). [ASCO abstract 7524]. J Clin Oncol. 2021;39(15 suppl). Doi: 10.1200/JCO.2021.39.15_suppl.7524
- Huang Q, Borra S, Li J, Wang L, Shrestha S, Sundaram M, Janjan N. Time to Next Treatment, Health Care Resource Utilization, and Costs Associated with Ibrutinib Use Among U.S. Veterans with Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma: A Real-World Retrospective Analysis. J Manag Care Spec Pharm. 2020 Oct;26(10):1266-1275. doi: 10.18553/jmcp.2020.20095. Epub 2020 Sep 3.
- Frei CR, Le H, McHugh D, Ryan K, Jones X, Galley S, Franklin K, Baus CJ, Tavera J, Janania-Martinez M, Gregorio D, Ananth S, Uribe R, Surapaneni P, Espinoza-Gutarra M, Song MM, Teng C, Obodozie-Ofoegbu OO, Nooruddin Z. Outcomes in chronic lymphocytic leukemia patients on novel agents in the US Veterans Health Administration System. Leuk Lymphoma. 2021 Feb 11:1-14. doi: 10.1080/10428194.2021.1876863. Epub ahead of print.
- By VashiDonsk at the English Wikipedia, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=2111159
- By Cancer Research UK – Original email from CRUK, CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=34334071