HOUSTON — While small-cell lung cancer has traditionally been treated as a single entity with generally poor results, that might be changing.
A study published in the journal Cancer Cell is the first to describe a comprehensive framework to classify SCLC into four unique subtypes, based on gene expression. Researchers from the University of Texas MD Anderson Cancer Center and colleagues also identified potential therapeutic targets for each type.1
SCLC is notoriously aggressive and resistant to treatment. The study, which received funding from DoD and other federal entities, noted that recent advances in immunotherapy and targeted therapy have improved survival for non-small cell lung cancer (NSCLC), but progress for improving survival in SCLC has been limited.
“For decades, small-cell lung cancer has been treated as a single disease, because the tumors all look similar under the microscope, even though they behave very differently,” said senior author Lauren Averett Byers, MD, associate professor of thoracic/head and neck medical oncology and senior author of the study. “Our study provides a transformative new system to define four major groups of small-cell lung cancer and, for the first time, an avenue for personalized treatment of the second most common type of lung cancer.”
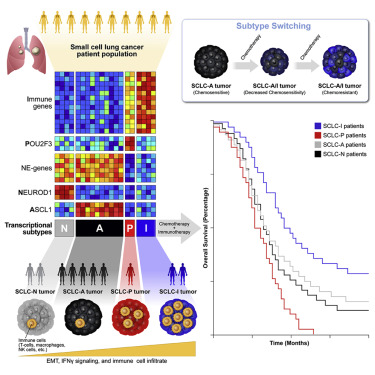
“Despite molecular and clinical heterogeneity, small cell lung cancer (SCLC) is treated as a single entity with predictably poor results,” study authors pointed out. They used tumor expression data and nonnegative matrix factorization to identify four SCLC subtypes “defined largely by differential expression of transcription factors ASCL1, NEUROD1, and POU2F3 or low expression of all three transcription factor signatures accompanied by an inflamed gene signature (SCLC-A, N, P, and I, respectively).”
Researchers determined that, in SCLC-I , the addition of immunotherapy to chemotherapy has the greatest benefit, while the other subtypes each have distinct vulnerabilities, including to inhibitors of PARP, Aurora kinases, or BCL-2.
For example, they wrote, “Cisplatin treatment of SCLC-A patient-derived xenografts induces intratumoral shifts toward SCLC-I, supporting subtype switching as a mechanism of acquired platinum resistance.”
Researchers posited that “matching baseline tumor subtype to therapy, as well as manipulating subtype switching on therapy, may enhance depth and duration of response for SCLC patients.”
Previous research identified three possible subtypes of SCLC based on transcription factors, but many tumors fell outside of those three groups. This study used data from a large set of tumor samples to confirm the three previously observed groups (A, N and P) but add a fourth (I) with a unique immune landscape.
While the first three groups are defined by activation of the ASCL1 (SCLC-A), NEUROD1 (SCLC-N) and POU2F3 (SCLC-P) genes, the fourth type, SCLC-I, is notable for an inflamed gene signature with a high expression of multiple immune genes. It also includes greater levels of genes indicating the presence of CD8-positive cytotoxic T cells.
More Efficacy
“Our paper shows that the inflamed group has a distinct biology and environment and tends to be more responsive to immunotherapy,” Byers said. “Identifying the inflamed group is very important, because so far there have not been any validated biomarkers for small-cell lung cancer that predict which patients get the most benefit from immunotherapy.”
The authors suggested their study could help explain why different classes of drugs have more efficacy in specific subtypes. They found that:
- SCLC-I was most sensitive to immune checkpoint blockade,
- SCLC-A to BCL2 inhibitors,
- SCLC-N to Aurora kinase inhibitors and
- SCLC-P to PARP inhibitors.
“Immunotherapy plus chemotherapy is currently the backbone of treatment for all advanced small-cell lung cancer patients, but not all patients experience the same benefit,” added Carl Gay, MD, PhD, assistant professor of thoracic/=head and neck medical oncology and lead author of the study. “Our results provide an opportunity to think about immunotherapy approaches that are specific to the inflamed group, which has a very different microenvironment, separately from combination approaches that might activate the immune response in the other three groups.”
Identification of the four groups was aided by reviewing previously published data from 81 SCLC patients with surgically resected tumors. Most patients in that data set had atypical early-stage disease, even though SCLC is so aggressive, it’s most often diagnosed at an advanced stage.
To validate the four subtypes in late-stage disease, researchers analyzed data from 276 SCLC patients enrolled in the Phase III IMpower133 clinical trial, which established the current standard of care for advanced SCLC and represents the largest available SCLC data set to date.
“Looking at the bigger data set of what a more typical patient looks like, the four major groups came out very clearly again, including the novel inflamed group we identified,” Byers said. “We also showed that you don’t have to use the full 1,300 gene panel. We have developed immunohistochemistry tests that we’re working toward adapting for the clinic to more quickly and easily classify SCLC tumors.”
The research also came up with some possible solutions for dealing with acquired resistance to treatment. In an effort to find out if “subtype switching” could be causing resistance, the study authors employed single-cell RNA sequencing to evaluate tumor evolution in a series of patient-derived SCLC models. The study signaled that SCLC-A tends to switch to SCLC-I after being treated with chemotherapy, which could contribute to treatment resistance.
The ultimate goal, according to the authors, is to make SCLC treatment more personalized and effective.
“Now we can develop more effective strategies for each group in clinical trials, taking into account that they each have different biology and optimal drug targets,” Byers said. “As a field, small-cell lung cancer is about 15 years behind non-small cell lung cancer’s renaissance of biomarkers and personalized therapies. This represents a huge step in understanding which drugs work best for which patients and gives us a path forward for personalized approaches for small-cell lung cancer.”
- Gay CM, Stewart CA, Park EM, Diao L, et. al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell. 2021 Mar 8;39(3):346-360.e7. doi: 10.1016/j.ccell.2020.12.014. Epub 2021 Jan 21.