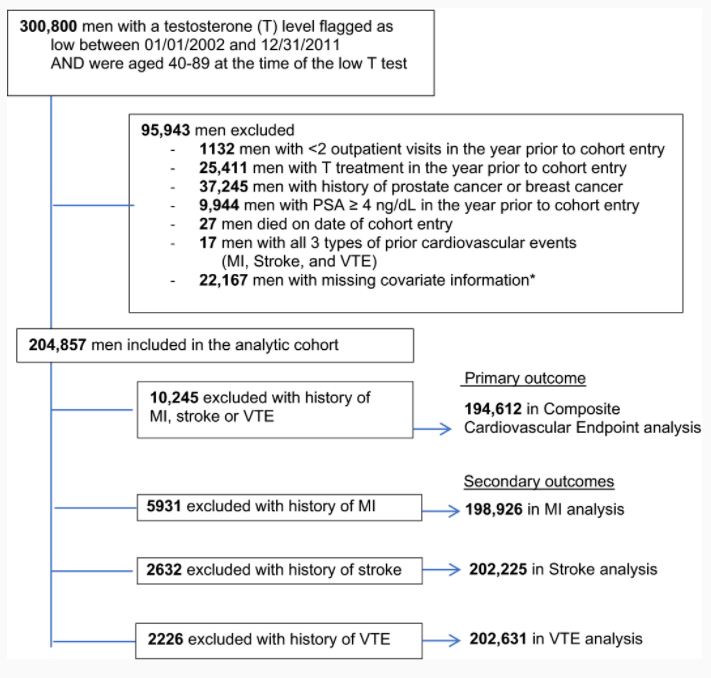
Study cohort inclusions and exclusions.
*Among 22 167 men with missing information: 20 957 were missing information on race, 1200 were missing information on BMI, 825 were missing information on region. These are overlapping groups, as some men were missing >1 covariate. BMI indicates body mass index; MI, myocardial infarction; PSA, prostate‐specific antigen; and VTE, venous thromboembolism.
SEATTLE — Hormone replacement therapy can have a number of beneficial effects—improving libido and sexual function, lifting energy and mood, increasing muscle mass and bone mineral density and decreasing fat mass—in men with low levels of testosterone, according to studies.
In 2000, thanks to ad campaigns targeting middle-aged men, prescriptions for testosterone therapy to treat symptoms related to low levels of the hormone (or “low T”) shot up, going far beyond men who, according to clinical guidelines, required treatment.
Then in 2013, observational studies reported a possible association between testosterone therapy and increased cardiovascular risk. Two years later, the U.S. Food & Drug Administration added warnings regarding cardiovascular risk in prescribing information for testosterone formulations. The number of men taking testosterone began to decline, yet subsequent studies produced conflicting results and the heart risks of testosterone treatment remain unclear.
“The conflicting results of prior observational studies may be due to cohorts that differed in age, prevalent cardiovascular disease, or medical morbidity. In addition, many studies had limited information on testosterone treatment (such as formulation, duration of treatment) and follow-up testosterone levels,” explained Molly M. Shores, MD, emeritus associate professor at the University of Washington in Seattle who also has been on staff at the VA Puget Sound Health Care System, also in Seattle.
Shores is the first author of a new paper published in the Journal of the American Heart Association that reexamines the possible link between cardiovascular events and testosterone treatment in an attempt to clarify the risks.1
The researchers collected data on more than 204,857 men with age-related low testosterone from the VHA and the Centers for Medicare and Medicaid Services. The participants were male veterans age 40 to 89 with low serum testosterone.
“All subjects had documented baseline low testosterone levels, which is the population in whom testosterone treatment might be indicated for,” said Shores. “In contrast, many [prior] studies had no information on baseline testosterone levels or gonadal status.”
The team also compiled detailed data on medical comorbidities—including kidney disease, chronic obstructive pulmonary disease, diabetes, high cholesterol, hypertension, major depression, obesity, sleep apnea, smoking, prevalent cardiovascular disease and other diagnoses—and adjusted for baseline medical comorbidity and changes in medical comorbidity throughout the study.
Instead of comparing men who had used testosterone to those who had never used testosterone, the researchers gathered detailed pharmacy data to categorize participants as “current users,” “former users” and “never users.”
The team compared current users to former users—who presumably had a previous indication for testosterone treatment—instead of comparing current-users to nonusers. This is important, Shores said, because testosterone-treated men may be healthier than untreated men, who may be at greater risk for cardiovascular events. In addition, distinguishing between current and former users “allowed us to ascertain intermittent and discontinued testosterone treatment and to assess if cardiovascular events occurred during periods of current, active testosterone use.”
Short Duration
Among the participants, they identified 82,555 men who had used testosterone therapy. Of those participants, 38% used transdermal testosterone patches, 40% used intramuscular testosterone injections, and 22% received both forms of testosterone (but usually not concurrently). The duration of the treatment was generally short, with a median cumulative treatment time of 4.8 months for transdermal testosterone and 11.1 months for intramuscular testosterone.
The team identified 12,645 total cardiovascular events but did not find a consistent link between testosterone use—whether transdermal or intramuscular—and an increased risk of these events, including myocardial infarction, ischemic stroke, or venous thromboembolism.
“This study provides some reassurance that there does not appear to be a significant risk for cardiovascular events, as no risks were detected in a large cohort of men with high medical morbidity and a high number of major cardiovascular events,” Shores said.
However, she said, “a large, randomized, double-blind, placebo-controlled trial is needed to definitively assess the cardiovascular risks of testosterone.” Such a trial is currently underway, but until the results are available, the study noted that clinicians should follow recommended guidelines regarding testosterone treatment and carefully review the potential risks and benefits.2
In addition, Shores said, future studies are needed to examine whether there are increased risks associated with high or low testosterone levels, whether testosterone injections are associated with increased cardiovascular events in the days immediately following the injection (when testosterone levels are at their highest) and whether subgroups of men are at increased risk for adverse outcomes due to advanced age or race.
Testosterone prescribing at the VHA followed national trends and increased substantially during fiscal years 2008-14. During that time period, testosterone was the 13th most commonly prescribed drug in the healthcare system, ranking slightly below cardiovascular medications, opioids, antidepressants and antipsychotics.
Questions about cardiovascular events in men taking the hormones—stemmed the tide somewhat, according to a report published in the Endocrine Society’s Journal of Clinical Endocrinology & Metabolism.3
Despite the slight decline in prescriptions, concerns continue on whether those prescriptions follow clinical guidelines and/or indications for which the pharmaceuticals have been approved.
A study team led by researchers from the Edith Nourse Rogers Memorial Veterans Hospital in Bedford, MA, and Boston University School of Public Health took a close look at exactly what was going on at the VHA in terms of testosterone prescribing. The review pinpointed characteristics of clinicians more and less likely to prescribe the hormones.
They determined that providers ranging in age from 31 to 60 years, with less experience in the VA and credentialed as medical doctors in endocrinology and urology, were more likely to prescribe testosterone. On the other hand, older providers, providers of longer VA tenure, and primary care providers were less likely to do so. The researchers pointed out that, while VHA endocrinologists were more likely to prescribe testosterone, they also were more likely to obtain an appropriate work-up before prescribing, compared to primary care providers.
- Shores, Molly M., Walsh, Thomas J., Korpak, Anna, Krakauer, Chloe, Forsberg, Christopher W., Fox, Alexandra E., Moore, Kathryn P., Heckbert, Susan R., Thompson, Mary Lou, Smith, Nicholas L., Matsumoto, Alvin M. Association Between Testosterone Treatment and Risk of Incident Cardiovascular Events Among US Male Veterans With Low Testosterone Levels and Multiple Medical Comorbidities. Journal of the American Heart Association. Published August 21, 2021. DOI: 10.1161/JAHA.120.020562
- The TRAVERSE study: https://www.traverse-study.com/
- Jasuja GK, Bhasin S, Rose AJ, Reisman JI, Hanlon JT, Miller DR, Morreale AP, Pogach LM, Cunningham FE, Park A, Wiener RS, Gifford AL, Berlowitz DR. Provider and Site-Level Determinants of Testosterone Prescribing in the Veterans Healthcare System. J Clin Endocrinol Metab. 2017 Sep 1;102(9):3226-3233. doi: 10.1210/jc.2017-00468. PubMed PMID: 28911150.
| No prevalent cardiovascular disease* | Prevalent cardiovascular disease* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Transdermal testosterone | PY/1000 | Events | IR | Unadjusted HR | Adjusted HR† | PY/1000 | Events | IR | Unadjusted HR | Adjusted HR† | |
| Composite cardiovascular end point‡ | Former | 71.5 | 688 | 9.62 | 1.00 (ref) | 1.00 (ref) | 68.3 | 1415 | 20.73 | 1.00 (ref) | 1.00 (ref) |
| Current | 20.7 | 180 | 8.68 | 0.89 (0.75–1.05) | 0.89 (0.76–1.05) | 16.1 | 254 | 15.74 | 0.74 (0.64–0.84) | 0.80 (0.70–0.91)§ | |
| No use | 332.2 | 3424 | 10.31 | 1.07 (0.98–1.16) | 1.02 (0.94–1.11) | 299.0 | 6684 | 22.35 | 1.06 (1–1.12) | 1.03 (0.97–1.09) | |
| MI | Former | 71.5 | 359 | 5.02 | 1.00 (ref) | 1.00 (ref) | 73.1 | 851 | 11.64 | 1.00 (ref) | 1.00 (ref) |
| Current | 20.7 | 95 | 4.58 | 0.91 (0.73–1.15) | 0.92 (0.73–1.15) | 17.2 | 151 | 8.78 | 0.74 (0.62–0.88) | 0.80 (0.67–0.95)§ | |
| No use | 332.2 | 1860 | 5.60 | 1.12 (1–1.25) | 1.06 (0.95–1.19) | 319.8 | 4170 | 13.04 | 1.11 (1.03–1.19) | 1.07 (0.99–1.15) | |
| Stroke | Former | 71.5 | 168 | 2.35 | 1.00 (ref) | 1.00 (ref) | 76.6 | 450 | 5.88 | 1.00 (ref) | 1.00 (ref) |
| Current | 20.7 | 45 | 2.17 | 0.93 (0.67–1.3) | 0.96 (0.69–1.34) | 17.7 | 86 | 4.85 | 0.82 (0.65–1.03) | 0.90 (0.72–1.14) | |
| No use | 332.2 | 865 | 2.60 | 1.12 (0.95–1.32) | 1.06 (0.90–1.25) | 334.5 | 2182 | 6.52 | 1.10 (1–1.22) | 1.05 (0.94–1.16) | |
| VTE | Former | 71.5 | 172 | 2.40 | 1.00 (ref) | 1.00 (ref) | 76.8 | 386 | 5.03 | 1.00 (ref) | 1.00 (ref) |
| Current | 20.7 | 42 | 2.03 | 0.78 (0.56–1.1) | 0.75 (0.53–1.05) | 17.6 | 64 | 3.63 | 0.66 (0.5–0.86) | 0.72 (0.55–0.94)§ | |
| No use | 332.2 | 739 | 2.22 | 0.89 (0.75–1.05) | 0.86 (0.73–1.02) | 337.9 | 1622 | 4.80 | 0.91 (0.81–1.02) | 0.93 (0.83–1.04) | |
| Intramuscular testosterone | |||||||||||
| Composite cardiovascular end point‡ | Former | 59.1 | 652 | 11.03 | 1.00 (ref) | 1.00 (ref) | 58.7 | 1250 | 21.29 | 1.00 (ref) | 1.00 (ref) |
| Current | 37.8 | 379 | 10.03 | 0.89 (0.79–1.01) | 0.91 (0.80–1.04) | 31.1 | 596 | 19.16 | 0.88 (0.8–0.98) | 0.98 (0.89–1.09) | |
| No use | 327.6 | 3261 | 9.96 | 0.89 (0.81–0.96) | 0.82 (0.75–0.89) | 293.6 | 6507 | 22.16 | 1.03 (0.97–1.09) | 0.96 (0.90–1.02) | |
| MI | Former | 59.1 | 349 | 5.90 | 1.00 (ref) | 1.00 (ref) | 62.7 | 774 | 12.34 | 1.00 (ref) | 1.00 (ref) |
| Current | 37.8 | 211 | 5.58 | 0.93 (0.79–1.11) | 0.95 (0.80–1.13) | 32.7 | 364 | 11.14 | 0.89 (0.79–1.01) | 0.99 (0.87–1.12) | |
| No use | 327.6 | 1754 | 5.35 | 0.89 (0.79–1) | 0.84 (0.75–0.94) | 314.6 | 4034 | 12.82 | 1.03 (0.95–1.11) | 0.97 (0.89–1.04) | |
| Stroke | Former | 59.1 | 158 | 2.67 | 1.00 (ref) | 1.00 (ref) | 65.8 | 411 | 6.25 | 1.00 (ref) | 1.00 (ref) |
| Current | 37.8 | 90 | 2.38 | 0.90 (0.69–1.17) | 0.95 (0.73–1.23) | 34.2 | 162 | 4.73 | 0.77 (0.64–0.92) | 0.87 (0.73–1.05) | |
| No use | 327.6 | 830 | 2.53 | 0.95 (0.8–1.13) | 0.86 (0.72–1.02) | 328.8 | 2145 | 6.52 | 1.05 (0.95–1.17) | 0.94 (0.84–1.04) | |
| VTE | Former | 59.1 | 155 | 2.62 | 1.00 (ref) | 1.00 (ref) | 66.3 | 306 | 4.62 | 1.00 (ref) | 1.00 (ref) |
| Current | 37.8 | 82 | 2.17 | 0.77 (0.59–1.01) | 0.77 (0.59–1.00) | 34.2 | 146 | 4.27 | 0.87 (0.71–1.06) | 0.97 (0.79–1.18) | |
| No use | 327.6 | 716 | 2.19 | 0.79 (0.66–0.94) | 0.71 (0.60–0.85) | 331.9 | 1620 | 4.88 | 1.02 (0.9–1.15) | 0.94 (0.83–1.07) | |

