VA researchers solved a mystery involving younger veterans who developed unusual and deadly cancers. They determined that patients currently using certain drugs, thiopurines, had triple the risk of myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) on an adjusted basis compared to those never exposed. The risks resolved with discontinuation.
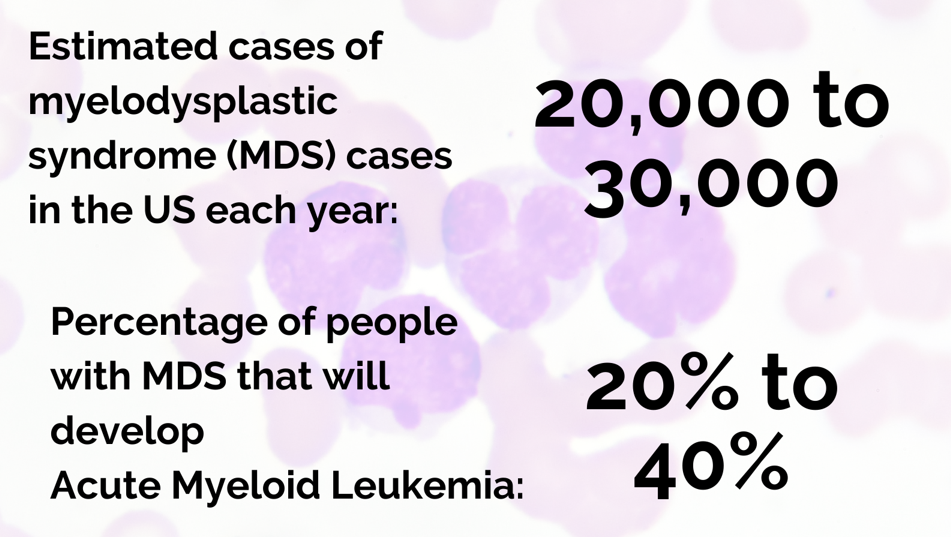
Source: National Cancer Institute.
PHILADELPHIA — The researchers at the VA may not wear Sherlock Holmes’ deer-stalker cap or Colombo’s wrinkled raincoat, but their work as investigators requires the same tireless detective work and determination to identify suspects and find the bad actor.
In a case in point, a team at the Corporal Michael J. Crescenz VAMC and the University of Pennsylvania Perelman School of Medicine, both in Philadelphia, had cases of an unusual and deadly cancer and a suspect drug with an extensive rap sheet but no solid evidence linking it to the cancer.
The link between the suspect, the thiopurine class of drugs, and nonmelanoma skin cancers and lymphoma has been well documented. Investigation into their connection to urinary tract cancer is underway. The thiopurines azathioprine, mercaptopurine, and thioguanine are commonly used to manage the symptoms of inflammatory bowel disease (IBD), both ulcerative colitis and Crohn’s disease. Could these drugs also be instigators in the development of myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) in the same patient population?
The investigators combined MDS and AML into one entity for their study because the myeloid stem cell lineage is affected in both conditions and because MDS, which is also called preleukemia, transforms into AML in about one-third of cases. MDS includes a range of disorders in which the bone marrow fails to produce enough healthy, fully functional blood cells. In AML, an excess number of myeloblasts or immature white blood cells interferes with the production of other types of blood cells and platelets. Both conditions occur at the rate of 4.3 to 4.5 per 100,000 patients years in the general population and have low survival rates, less than 50% at three years for MDS and 28% at five years for AML.
Previous studies of a potential connection between thiopurines and AML/MDS had been hampered by a small number of cases and an unusually young age for the patients, two problems the VA researchers overcame with access to the nationwide VA cohort. The cohort has a median age of 64 years, in line with the median age at diagnosis for AML (68) and MDS (71).
The team identified 56,314 veterans with a diagnosis of IBD over an 18-year period. Of those, 15,305 had ever been exposed to thiopurines (TP). The study followed patients for a median of 9.7 years. During the study period, 107 patients developed AML/MDS, for a crude incidence rate of 18.7 per 100,000.
Twenty-one veterans developed AML/MDS while receiving thiopurines, all of whom also developed incident leukopenia in the two years prior to AML/MDS diagnosis. The majority had sustained leukopenia in the lead up to their AML/MDS diagnosis. “This was a very significant finding that underlines the importance of monitoring WBC counts in patients on TPs, especially among the elderly,” the researchers said. “In light of our findings, if patients are found to have leukopenia while on TPs, a hematological consult should be considered for a possible bone marrow biopsy evaluation especially among the elderly population subgroup.”
Veterans currently using thiopurines who had a cumulative exposure of less than two years to the drugs had triple the risk of AML/MDS on an adjusted basis compared to those never exposed. Those who were currently using thiopurines and had more than two years of exposure had an adjusted risk 2.32 times that of individuals never exposed to the drugs. Past use of thiopurines was not associated with any increased risk of AML/MDS.
While older age predictably increased the risk of AML/MDS, there was “no significant multiplicative interaction between the age and the TP exposure status,” the team said. “However, it should be noted that because elderly have an increased absolute risk of developing AML/MDS, if they are prescribed a TP, the absolute risk increase is higher than that in the younger population.”
The team added a bit of good news from the study: the elevated risk of AML/MDS associated with exposure to thiopurines reverted to baseline withing six months of discontinuation.
Treatment Options in MDS
For those who develop MDS, recent developments provide more options for treatment. Many patients are eligible for watchful waiting as MDS can take an indolent course. Others require more aggressive treatment and frequent transfusions. While the only curative therapy is a blood marrow transplant, disease-modifying therapies can slow progression and extend survival for some patients without the toxicity of chemotherapy.
No new drugs for MDS gained U.S. Food and Drug Agency approval between the 2006 okay of lenalidomide and 2020, leaving patients and physicians to choose between three azanucleotides delivered as infusions. Azacitidine and decitabine can be used for all types of MDS; lenalidomide is indicated only for the 5q-syndrome type.
In the last two years, luspatercept, an infused erythroid maturation agent, gained approval for use in patients with anemia who have failed erythropoiesis-stimulating agent and need two or more transfusions in a two-month period. It is indicated for very low-risk to intermediate-risk MDS with ring sideroblasts.
Also in 2020, a tablet that combines decitabine and cedazuridine received the FDA green light, making it the first oral treatment for MDS. It is indicated for a broad range of MDS patients including previously treated and treatment naïve individuals with de novo or secondary MDS with refractory anemia, refractory anemia with ringed sideroblasts or refractory anemia with excess blasts. It is approved for patients in the intermediate-1, intermediate-2, and high-risk International Prognostic Scoring System groups.
Other agents are also on the horizon for MDS including venetoclax, which is already used in AML. Magrolimab, a macrophage immune checkpoint inhibitor, that appears promising in patients with TP53 mutations.
- Khan N, Patel D, Trivedi C, Kavani H, Pernes T, Medvedeva E, Lewis J, Xie D, Yang YX. Incidence of Acute Myeloid Leukemia and Myelodysplastic Syndrome in Patients With Inflammatory Bowel Disease and the Impact of Thiopurines on Their Risk. Am J Gastroenterol. 2021 Apr;116(4):741-747. doi: 10.14309/ajg.0000000000001058. PMID: 33982944.

