Already a presumptive condition for Vietnam-era veterans exposed to Agent Orange and military personnel who were at Camp Lejeune in the mid-20th century, follicular lymphoma is also one of the presumptive conditions associated with burn put for Gulf War era and post-9/11 veterans under the new PACT Act. Recent advances have provided a greater understanding of the biological changes that precede the development of FL, a type of non-Hodgkin’s lymphoma, and treatment options are improving.
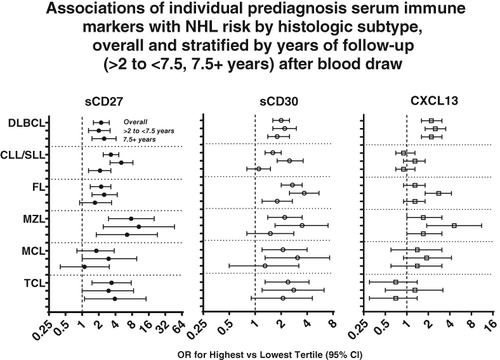
Click to Enlarge: Although prediagnostic circulating concentrations of the immune activation markers sCD27, sCD30 and CXCL13 have been associated with non-Hodgkin lymphoma risk, studies have been limited by sample size in associations with non-Hodgkin lymphoma subtypes. This pooled analysis of the three prediagnostic serum immune marker concentrations and non-Hodgkin lymphoma subtypes, the first of its kind, provides robust evidence implicating subclinical immune activation effects in the pathogenesis of diffuse large B cell lymphoma. The results also offer novel insights into the etiologies of rare lymphomas such as marginal zone and T cell lymphomas. Source: International Journal of Cancer
SILVER SPRING, MD — With the passage of the Sgt. 1st Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics Act of 2022 (PACT Act), Congress added follicular lymphoma to the presumptive conditions associated with burn pits and other exposures for Gulf War era and post-9/11 veterans. A type of non-Hodgkin lymphoma, the cancer was already a presumptive condition for Vietnam-era veterans exposed to Agent Orange and servicemembers exposed to contaminated water at Camp Lejeune from 1953-87.
Follicular lymphoma accounts for 10% of all non-Hodgkin lymphomas, and approximately 15,000 cases are diagnosed in the United States each year. While typically an indolent disease that might never require treatment in up 30% of patients, it transforms into a much more aggressive disease, typically diffuse large B-cell lymphoma, in about 2% of cases.
For most patients with follicular lymphoma (FL), however, the disease becomes a chronic condition with no cure. While initial therapy often produces a remission of two years or more, many patients will experience a relapse or recurrence that requires additional lines of therapy.
Recent advances have provided a greater understanding of the biological changes that precede the development of FL and may provide biomarkers for the disease. In addition, new therapies provide additional treatment options suitable for patients of varying health status, age and preferences.
B-cell Activation
Researchers at the Walter Reed Army Institute of Research in Silver Spring, MD, the National Cancer Institute in Rockville, MD, and other leading cancer centers, examined subtle alterations in immune function in search of biomarkers that could herald or presage development of non-Hodgkin lymphomas (NHL) including FL.1
Previous studies indicated that elevated levels of soluble CD27 and CD30 along with chemokine ligand-13 (CXCL13) were associated with high risk of NHL. “The precise time frame that would reflect disease-induced rather than etiologic effects in not known for most subtypes,” even though the differences in aggressiveness would suggest subtype-specific variability in early stages, the team said.
To provide clarity on the relationship, the researchers conducted a pooled analysis of eight nested case-control studies that focused on one or more of these markers. The analysis included 2,455 cases and 3,310 controls. The team found that the odds ratio associated with the biomarkers varied significantly between the subtypes. Levels of sCD27 were much more strongly associated with other subtypes of NHL than with follicular lymphoma, but the odds ratios associated with CXCL13 varied less across the subtypes and not at all for sCD30. Modeling the three immune markers simultaneously found sCD30 was only associated with FL and diffuse large B-cell lymphoma (DLBCL).
“We found sCD27 and sCD30 to be associated with [chronic lymphocytic leukemia/small lymphocytic lymphoma] and FL, respectively, independent of other markers, although both associations were approximately 50% weaker for cases diagnosed ≥7.5 years post phlebotomy than for cases diagnosed closer in time to the date of blood collection. These patterns may suggest a later-stage role in the development of CLL/SLL and FL for the biologic effects captured by these markers,” the researchers said. On the other hand, they noted, the study could not rule out that the markers indicated early-stage disease.
Another study examined the longitudinal changes in immune activation of these same biomarkers plus IL10 before a diagnosis of NHL in active-duty military personnel. Led by Lynn Levin, PhD, MPH, of Walter Reed, the researchers reached somewhat different conclusions in their study of changes in serum levels of the biomarkers in 142 servicemembers with FL and 211 with DLBCL, with each matched to two healthy controls.2
They found that increasing levels of sCD30 and CXCL13 started from the earliest collection times, while IL10 rose markedly closer to diagnosis. “These results suggest that sCD30, CXCL13, and IL10 may contribute to the etiology of FL and DLBCL and are potential biomarkers for these non-Hodgkin lymphoma subtypes,” Levin and her colleagues wrote. Like the previous team, however, they could not determine whether the increasing trajectories of these markers “may indicate early disease-induced effects or reflect the chronic stimulation of B-cells that promotes the development of FL and DLBCL subtypes.”
Shakeup in Treatment Options
While research into the earliest stages of FL may ultimately provide interventions that can prevent its development in future patients, advances in treatment options can help individuals who have the disease today.
Patients diagnosed with FL typically start treatment with immunochemotherapy or rituximab monotherapy. Those who do respond or relapse within 24 months have a worse prognosis and may benefit from autologous hematopoietic stem cell transplantation or treatment with a chimeric antigen receptor T-cell therapy. The U.S. Food and Drug Administration has approved three in the last two years: lisocabtagene maraleucel, axicabtagene ciloeucel, and tisagenlecleucel. Because of their toxicity, CAR-T therapies are most suitable for younger patients with aggressive disease.
Patients who relapse after 24 months will likely need multiple lines of therapy over the course of decades. Some drugs that have been used in this setting have been withdrawn in the last two years; others have gained approvals.
Notably, manufacturers are no longer supporting several phosphoinositide 3-kinase (PI3K) inhibitors and combinations for follicular lymphoma or announced they are pulling their applications for the indication, including umbralisib/obinutuzumab, umbralisib/ublituximab, umbralisib alone, duvelisib and idelalisib. Copanlisib is the only PI3K remaining on the market for FL.
Newer drugs approved for follicular lymphoma include tazemetostat for patients with mutated EZH2, about 20% of FL cases, and those with no other good options. An oral drug, it is less immunosuppressive than many other options and has minimal side effects, making it particularly appropriate for frail and elderly patients. Mosunetuzumab, a bispecific antibody that targets CD20 and CD3, is another new therapy for FL.
- Rhee J, Birmann BM, De Roos AJ, Epstein MM, Martinez-Maza O, et. al.. Circulating immune markers and risks of non-Hodgkin lymphoma subtypes: A pooled analysis. Int J Cancer. 2023 Mar 1;152(5):865-878. doi: 10.1002/ijc.34299. Epub 2022 Oct 5. PMID: 36151863; PMCID: PMC9812887.
- Levin LI, Ramirez CM, Liao EL, Guo H, et. al. Longitudinal Changes in Immune Activation Serum Biomarkers Prior to Diagnosis and Risk of B-cell NHL Subtypes. Cancer Epidemiol Biomarkers Prev. 2023 Feb 6;32(2):233-241. doi: 10.1158/1055-9965.EPI-22-0247. PMID: 36409490; PMCID: PMC9905313.
