Unlike veterans with a variety of types of lymphoma, the expanded list of presumptive conditions for the PACT Act did not include acute myeloid leukemia or other types of leukemia. Because AML occurs primarily in older adults, with an average age at diagnosis of 68, it remains a challenge for clinicians treating veterans receiving care from the VHA. The good news, according to recent studies, is that new combinations of treatment have now come online for older patients.
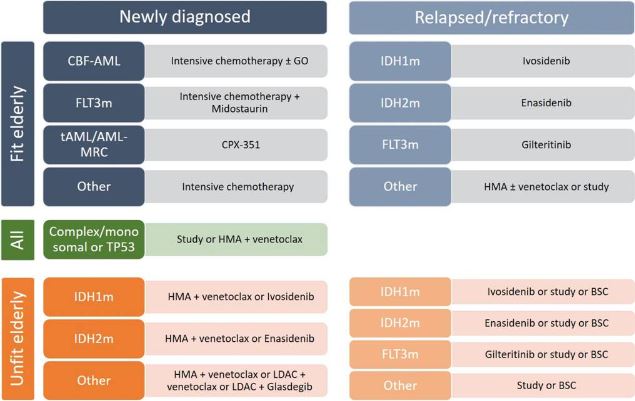
Click to Enlarge: Purposed treatment algorithm for elderly AML patients. CBF-AML, core-binding factor acute myeloid leukemia; FLT3m, FMS-like tyrosine kinase mutated; tAML, therapy-related AML; AML-MRC, AML with myelodysplasia-related changes; GO, gemtuzumab ozogamicin; HMA, hypomethylating agent; IDH1m, isocitrate dehydrogenase 1 mutated; IDH2m, isocitrate dehydrogenase 2 mutated; LDAC, low-dose cytarabine; BSC, best supportive care. Source: National Library of Medicine
CLEVELAND — Despite the recent expansion of the presumptive cancers related to burn pit exposure, the VA has not included any types of leukemia, leaving Gulf War and post-9/11 veterans with acute myeloid leukemia (AML) in the same position as their fellows who served in Vietnam.
Previous action determined that all adult leukemias, aplastic anemia and other myelodysplastic syndromes, multiple myeloma and non-Hodgkin lymphomas were presumptive conditions for veterans exposed to contaminated water at Marine Corps Base Camp Lejeune or Marine Corps Air Station New River, NC.
The presumptive conditions determinations for exposure to Agent Orange and burn pits took a more granular approach. For Vietnam veterans, a limited number of blood cancers were individually evaluated and covered, including chronic b-cell leukemia, Hodgkin disease, multiple myeloma and non-Hodgkin lymphoma. For burn pits, all lymphomas were included but no forms of leukemia, although the PACT Act added coverage for monoclonal gammopathy of unknown significance (MGUS); patients with MGUS have an eightfold statistically increased risk of developing AML/myelodysplastic syndromes.
In a recent presentation sponsored by the Association of VA Hematology/Oncology (AVAHO), Timothy O’Brien, MD, section chief of hematology at the Louis Stokes Cleveland VAMC discussed findings from a number of studies that showed an association between benzene exposure and AML. Benzene is a metabolite of the highly carcinogenic dioxin 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a common contaminant of Agent Orange. TCDD and benzene have been shown to induce chromosomal abnormalities common in patients with AML.
Further, data from industrial accidents and manufacturing processes demonstrated that high serum levels of TCDD correlated with higher risk for AML, leading the U.S. Agency for Toxic Substances and Disease Registry to conclude 15 years ago that “[e]pidemiological studies and case reports provide clear evidence of a causal relationship between occupational exposure to benzene and benzene-containing solvents and the occurrence of acute myelogenous leukemia.”
Still, clinical studies documenting a link between Agent Orange and AML have not been published. O’Brien attributed the lack of studies to both a variability in the tracking of cases of AML and the “long latency period between the Agent Orange exposure and development of AML and so a lot of veterans may have passed away from other causes” before AML develops or is diagnosed.
Despite a lack of dioxin exposure assessments in Vietnam era veterans, the “VA acknowledges that Agent Orange was contaminated by TCDD or dioxins and benzenes, which are known carcinogens,” O’Brien said. “Yet veterans of Camp Lejeune who were exposed to benzene contaminated water, who went on to develop AML qualify for service-connection benefit.”
Elderly Veterans With AML
Regardless of the determination of a service connection, a significant number of veterans who receive care through the VA have AML. For them, accessing a treatment that keeps the disease at bay is their highest priority.
The National Cancer Institute estimates that more than 73,000 people in the U.S. have AML and about 20,000 new cases will be diagnosed this year. The disease will kill an estimated 11,540 people in 2023.
The advances in stem cell transplantation and intensive chemotherapy (IC) that have increased the five-year survival rate of younger patients with AML have not reached the older patients who more typically develop the disease, who often have comorbidities and frailty that preclude such aggressive treatments.
“Recently, the addition of venetoclax to [hypomethylating agents (HMAs)] was shown to strongly improve outcome for older, medically non-fit AML patients, to such an extent that choosing the optimal treatment has become challenging,” noted researchers in a recent review. The review discussed the advantages and disadvantages of treatments for AML, including venetoclax, glasdegib, enasidenib, ivosidenib, gilteritinib, midostaurin, gemtuzumab ozogamicine, CC-486, and CPX-351.1
“The current standard for the treatment of elderly (> 75 years) and unfit AML is the combination of HMA and venetoclax. It has demonstrated excellent activity with favorable safety, even in frail patients,” the authors said. “Studies suggest that complete remission rates attained by these combinations may approach those of IC, and it is therefore often hypothesized that HMA with venetoclax should also be the preferred frontline treatment in elderly AML patients (60-75 years) who are fit for IC treatment.”
A number of recent studies have built off of this base to see which HMA is most effective with venetoclax.
One such study involving researchers from the VA Northern California Healthcare System in Sacramento assessed the safety and efficacy of decitabine or azacytidine with venetoclax in patients with relapsed/refractory and secondary AML (sAML). “Unlike the prior literature, which showed a 0% response rate and 0% 6-month survival in patients with sAML, we found an ORR of 50%, which was not significantly different from our response rate in de novo AML. Additionally, 45% of our sAML patients were alive 12 months, and 22% survived to at least 24 months in this high-risk population,” they said.2
Further, they found that the “use of HMA/Ven as a bridge to HSCT was successful in several patients with deeper responses who remained in remission at the end of follow-up. This regimen may represent a viable means of low-intensity re-induction prior to HSCT consolidation.”
Other researchers conducted a study evaluated a new HMA, OR21, which can be administered orally, unlike azacytidine or decitabine which are typically administered parenterally because of degradation by cytidine deaminase, although orally bioavailable forms (CC-486 and ASTX727) are available. They found that OR21 plus venetoclax “synergistic antileukemia effects in vitro and vivo, suggesting that the combination of OR2100 plus Ven is a promising candidate oral therapy for AML.”
- de Leeuw DC, Ossenkoppele GJ, Janssen JJWM. Older Patients with Acute Myeloid Leukemia Deserve Individualized Treatment. Curr Oncol Rep. 2022 Nov;24(11):1387-1400. doi: 10.1007/s11912-022-01299-9. Epub 2022 Jun 2.
- Tenold ME, Moskoff BN, Benjamin DJ, Hoeg RT, Rosenberg AS, Abedi M, Tuscano JM, Jonas BA. Outcomes of Adults With Relapsed/Refractory Acute Myeloid Leukemia Treated With Venetoclax Plus Hypomethylating Agents at a Comprehensive Cancer Center. Front Oncol. 2021 Mar 11;11:649209. doi: 10.3389/fonc.2021.649209.
- Kamachi K, Ureshino H, Watanabe T, Yoshida-Sakai N, Fukuda-Kurahashi Y, Kawasoe K, Hoshiko T, Yamamoto Y, Kurahashi Y, Kimura S. Combination of a New Oral Demethylating Agent, OR2100, and Venetoclax for Treatment of Acute Myeloid Leukemia. Cancer Res Commun. 2023 Feb 21;3(2):297-308. doi: 10.1158/2767-9764.CRC-22-0259.