About 9,000 Veterans Were Prescribed Drug for Hyperkalemia
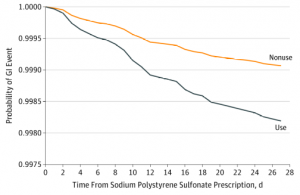
LEGEND: 30-Day Probability of Gastrointestinal (GI) Injury Requiring Hospitalization or Emergency Department Visit Associated With Sodium Polystyrene Sulfonate Use Compared With NonuseResults presented are for the matched analysis of sodium polystyrene sulfonate users to nonusers on the logit of the high-dimensional propensity score (±0.2 of the standard deviation) and the following: age, sex, diabetes, congestive heart failure, prior acute kidney injury, chronic dialysis, history of hyperkalemia, previous nephrologist visit, medication use (β-blocker or renin-angiotensin-aldosterone system blockade), and index date (within 1 year). Additional adjustment was for place of residence.
OTTAWA, ONTARIO — Continuing concerns are being raised about the gastrointestinal safety of sodium polystyrene sulfonate, which is commonly prescribed for the treatment of hyperkalemia.
The issue is of special concern at the VA, where patients tend to be older with more comorbidities. A recent report suggested that about 8,000 VA patients were being treated with sodium polystyrene sulfonate.
A study published in JAMA Internal Medicine recently sought to assess the risk of hospitalization for adverse GI events associated with sodium polystyrene sulfonate use in patients of advanced age. “The use of sodium polystyrene sulfonate is associated with a higher risk of hospitalization for serious adverse GI events,” study authors from the University of Ottawa concluded. “These findings require confirmation and suggest caution with the ongoing use of sodium polystyrene sulfonate.”1
Commentators from the San Francisco VAMC and the University of California San Francisco explained why the findings are so important for pharmacists and other health professionals.2
“In addition to very weak evidence for positive outcomes, there is mounting evidence of harm caused by this drug,” wrote the San Francisco VAMC’s Deborah Grady, MD, MPH, who also is the deputy editor of JAMA Internal Medicine, and her colleague at UCSF Monica Parks, MD.
Grady and Parks noted the following history:
-
As early as the 1980s, cases of intestinal necrosis associated with sodium polystyrene sulfonate in sorbitol, the typical method of administration, were reported;
-
In 2009, the FDA warned of the risk of intestinal necrosis with administration of sodium polystyrene sulfonate in sorbitol and recommended avoiding the combination of these medications in postoperative patients and those with intestinal obstruction or chronic bowel disease, including constipation.
-
In a literature review published in 2010, intestinal necrosis was described only as a complication of the combination of sodium polystyrene sulfonate and sorbitol; in particular, 70% sorbitol and sodium polystyrene sulfonate preparations without sorbitol were reported to be safe.
The editorialists pointed out that the mechanism of action leading to intestinal necrosis was assumed to be related to hyperosmotic load from sorbitol leading to adenosine triphosphate–dependent sodium-potassium pump dysfunction, alteration of cellular membrane transport systems, and reduced intestinal blood flow. Comorbidities such as intestinal obstruction, diabetes or vascular disease were thought to heighten the risk.
“More recently, however, case reports of intestinal necrosis following sodium polystyrene sulfonate administration without sorbitol have also emerged,” the commentary stated. “A systematic review of 48 cases of intestinal necrosis after drug administration found 17 cases among patients not taking sorbitol, suggesting that sodium polystyrene sulfonate alone could also cause this complication.”
The recent population-based, retrospective matched cohort study focused on eligible adults older than age 66 who received sodium polystyrene sulfonate from April 1, 2003, to September 30, 2015, in Ontario, Canada.
Defined as the primary outcome was a composite of adverse GI events—hospitalization or emergency department visit with intestinal ischemia/thrombosis, GI ulceration/perforation or resection/ostomy—within 30 days of initial sodium polystyrene sulfonate prescription.
From more than 1.8 million eligible adults, 27,704 patients were dispensed sodium polystyrene sulfonate; participants had a mean age of 78.5 and were mostly, 54.7%, male. For the study, 20,020 sodium polystyrene sulfonate users were matched to an equal number of nonusers.
Continue Reading:
