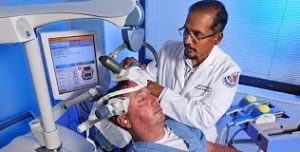
Prasad Padala, MD, MS, demonstrates how repetitive transcranial magnetic stimulation works with the help of a staff member at the Little Rock, AR, VAMC. Photo by Jeff Bowen
PALO ALTO, CA — Transcranial magnetic stimulation, or TMS, is a technique that uses strong magnetic pulses to stimulate regions of the brain, producing electric currents that may affect neuron activity. In 2008, this noninvasive technique was approved by the U.S. Food and Drug Administration for treatment-resistant depression; since then, it has also been approved for certain migraine headaches and obsessive-compulsive disorder. Now, scientists are investigating whether TMS could be beneficial for patients with neurological conditions including Alzheimer’s disease.1
Although several published studies suggest repetitive transcranial magnetic stimulation (a series of TMS sessions performed over time, known as rTMS) may improve cognition, researchers have not previously examined whether rTMS is effective in typical elderly populations with multiple medical comorbidities, such as those commonly seen in veterans. A new pilot study, published in The Journal of Alzheimer’s Disease, aims to shed more light on how well rTMS works in this population.2
There are almost nine million veterans aged 65 and older, and they are at higher risk for Alzheimer’s disease compared with the general population. There are likely many factors for veterans’ increased risk, said study author Kaci Fairchild, a clinical psychologist in the VA Palo Alto Health Care System and clinical associate professor at Stanford University School of Medicine.3
“Veterans experience a unique combination of military, lifestyle and health-related risk factors that increases their risk of developing dementia,” said Fairchild. “These unique risk factors in turn may impact the way that veterans respond to medical intervention.”
The researchers recruited 26 veterans aged 55 and older through outpatient and specialty care clinics in the VA Palo Alto Health Care System. All participants met the criteria for having mild or major neurocognitive disorder, likely due to Alzheimer’s. The most common comorbidities were hyperlipidemia, Type 2 diabetes, hypertension and obstructive sleep apnea. If patients were taking medications (including drugs approved to treat cognitive impairment), the researchers asked them to remain on the same dose throughout the study and follow-up period.
The veterans were randomly assigned to one of two groups. Half received active rTMS treatment, while the other half received sham (i.e., placebo) treatment. All subjects underwent 20 sessions over a four-week period. (Active rTMS was delivered at 10 Hz rTMS at the left dorsolateral prefrontal cortex with intensity of 120% resting motor threshold.) The researchers measured how well each group performed on assessments of memory, language, verbal fluency and executive functions before treatment, at the end of treatment and four months after the last rTMS session.
Although rTMS was well tolerated with no significant side effects, the study revealed that the treatment had mixed effects on participants’ cognitive testing results. Participants in the active rTMS group tended to have better memory recall—as has also been reported in previous studies—but performed slightly worse on verbal fluency. Interestingly, the positive effect on memory seemed to be even more pronounced four months after treatment.
“It is encouraging that our study showed rTMS may positively impact learning and memory in a group of veterans with mild cognitive impairment and early Alzheimer’s disease,” said Fairchild, although she stressed that the results are preliminary in nature. “The findings regarding the impact of rTMS on other cognitive functions were unexpected and highlight the necessity of conducting a larger study to verify the results of our pilot study. The bottom line is that we need to develop a deeper understanding on how rTMS impacts our brain and our cognitive function.”
This is the first study to highlight the potential benefit of rTMS for veterans with mild cognitive impairment and early Alzheimer’s disease. And, while the results are promising, the clinical implications are still unclear. In addition to increasing the size of the trial, lengthening the course of treatment and/or the monitoring period could allow researchers to more fully investigate the optimal dose and duration of rTMS treatment.
- FDA permits marketing of transcranial magnetic stimulation for treatment of obsessive compulsive disorder. U.S. Food & Drug Administration. Published August 17, 2018. https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-transcranial-magnetic-stimulation-treatment-obsessive-compulsive-disorder
- Cheng, Jauhtai; Fairchild, J. Kaci; McNerney, Margaret W.; Noda, Art; et al. Repetitive Transcranial Magnetic Stimulation as a Treatment for Veterans with Cognitive Impairment and Multiple Comorbidities. The Journal of Alzheimer’s Disease. Published December 21, 2021. DOI: 10.3233/JAD-210349.
- Veteran population. https://www.va.gov/vetdata/veteran_population.asp
