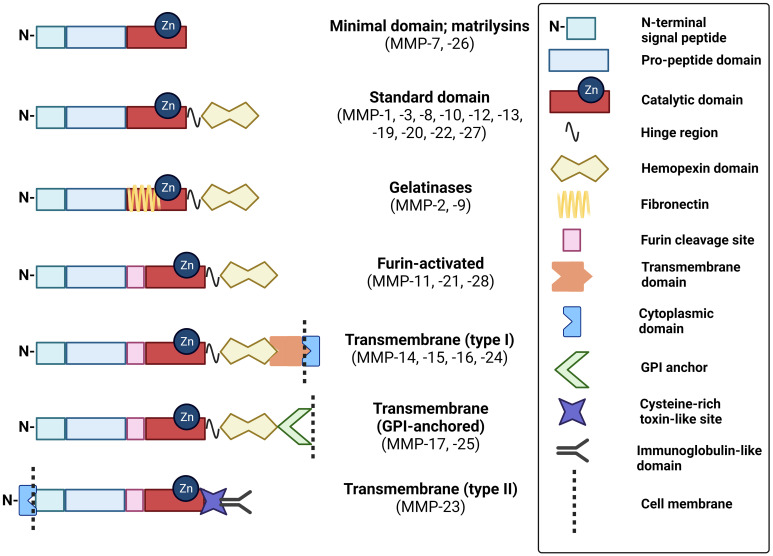
Click to Enlarge: Schematic of MMP domain structures. These schematics of MMP subtypes illustrate the relative distribution of key domains that modulate their structure and function. GPI, glycophosphatidylinositol. Source: Frontiers in Oncology Created with BioRender.com
BALTIMORE — An important research focus in colorectal cancer concerns the enzymes comprising the 24-member matrix metalloproteinase (MMP) family of zinc- and calcium-dependent endopeptidases. Those play key roles in extracellular matrix degradation, a requirement for colon tumor expansion, invasion and metastasis.
That is especially important, according to a recent report in Frontiers in Oncology, because colorectal cancer (CRC) remains a major cause of morbidity and mortality. “Therapeutic approaches for advanced CRC are limited and rarely provide long-term benefit,” noted researchers from the University of Maryland School of Medicine and the VA Maryland Healthcare System in Baltimore.1
The study team pointed out, “Compared to sporadic CRC, less is known regarding the molecular mechanisms and the role of MMPs in the development and progression of colitis-associated cancer (CAC)—CRC on a background of chronic inflammatory bowel disease (IBD)—primarily ulcerative colitis and Crohn’s disease.”
To remedy that, they reviewed data regarding the role of MMPs in the development and progression of CAC. “We sought to identify promising prognostic and therapeutic opportunities and novel lines of investigation,” according to the researchers. “A key observation is that, since MMPs may be more active in early phases of CAC, using MMPs as biomarkers of advancing neoplasia and as potential therapeutic targets for adjuvant therapy in those with advanced stage primary CAC rather than overt metastases may yield more favorable outcomes.”
More than 66,000 veterans have an IBD diagnosis, and the latest study on the increase in incidence showed that the prevalence of Crohn’s disease (CD) and ulcerative colitis (UC) increased twofold to threefold among VA healthcare users between 1998 and 2009.2
Background information in the article explained that colitis increases the risk of developing CAC due to the effect of chronic inflammation. That underscores the importance of understanding the pathology of chronic inflammation-induced CRC, according to the study team.
Chronic Inflammation
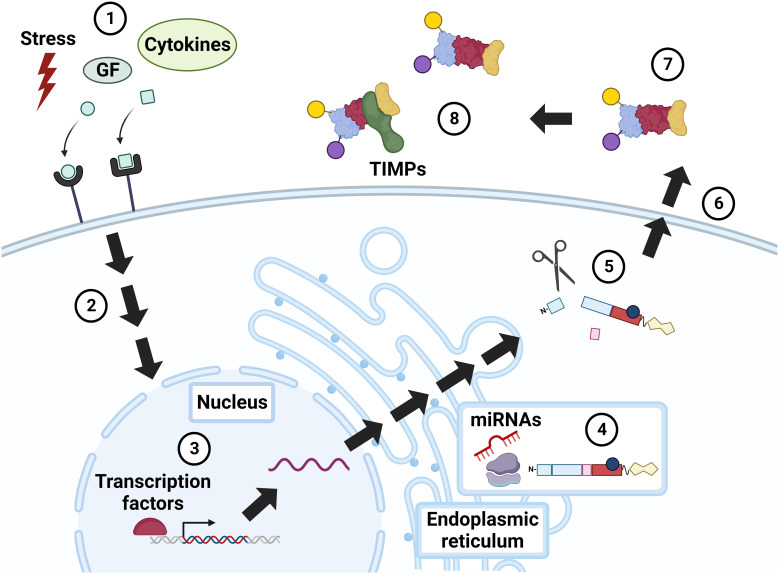
Click to Enlarge: Regulation of MMP activity. Constitutive MMP expression is augmented in response to external stimuli, e.g., physiochemical stress, growth factors (GF), and cytokines (1), that incite intracellular signaling via mitogen activated protein kinase (MAPK), NF-kB, Smad, and others (2). MMP expression is further modulated by transcription factors, notably AP-1 (3). MMP RNA is translated into a pre-propeptide (4), a process that can be up- and down-regulated by microRNAs (miRNAs). Cleavage of the N-terminal signal peptide in the endoplasmic reticulum (ER) yields the pro-MMP zymogen, which may undergo intracellular (e.g., cleavage by furin) and extracellular (e.g., cleavage by plasmin) modification (5). Cellular release of MMPs is also regulated by stress, growth factors, and cytokines (6). Extracellular MMPs are activated by post-translational modifications (7), or by the action of other, membrane-bound MMPs. MMP activity is also modulated by complexes formed with inhibitors, i.e., TIMPs and α2-macroglobulin (8). GF, growth factors; TIMP, tissue inhibitor of metalloproteinase. Source: Frontiers in Oncology Created with BioRender.com
“Although previously thought to participate only in ECM degradation, MMPs are now recognized to be directly involved in the development and sustenance of inflammatory states, including those that underlie both Crohn’s and ulcerative colitis,” the authors advised. “Yet, despite advances reviewed here, large gaps in knowledge exist regarding the role MMPs play in both CAC and the chronic inflammation that precedes and promotes CAC. There is a concordant lack of information regarding the potential utility of MMP inhibitors to treat these disorders.”
The researchers discussed how upregulation of specific MMPs, such as MMP-1, -2, -3, and -7, in intestinal inflammation and IBD “implicates not only their contribution to the development of CAC but also their potential as biomarkers to identify increasing dysplasia and incipient neoplasia. In contrast, other MMPs, like MMP-10, appear to be protective, auguring a more favorable prognosis.”
The report stated that some, such as MMP-9, “play seemingly context-dependent dual pro-inflammatory and anti-tumorigenic roles. Thus, prospectively testing the value of individual MMPs or a panel of MMPs in blood and tissue samples to gauge their value as biomarkers for dysplasia and incipient neoplasia appears to be a worthy avenue of investigation.”
All of this is an argument for continuing research focused on unraveling the pathways by which different MMPs exert their actions, according to the study team. “For example, further work is needed to unravel the pathways that modulate selective MMP upregulation after muscarinic agonism in colon cancer, how this interplay contributes to an invasive phenotype and its importance for the development and progression of CAC. Another potential avenue is to use this information to develop a panel of MMPs that can be tested in primary tumors to gauge the potential for advancing disease, the need for adjuvant therapy and the likelihood of response to conventional chemotherapeutic regimens.”
The researchers also emphasized the need for more understanding of the growing therapeutic applications of MMP inhibitors, especially as they relate to the management of IBD and CAC in those with an MMP profile predictive of aggressive disease.
- Sampaio Moura N, Schledwitz A, Alizadeh M, Patil SA, Raufman JP. Matrix metalloproteinases as biomarkers and therapeutic targets in colitis-associated cancer. Front Oncol. 2024 Jan 15;13:1325095. doi: 10.3389/fonc.2023.1325095. PMID: 38288108; PMCID: PMC10824561.
- Hou JK, Kramer JR, Richardson P, Mei M, El-Serag HB. The incidence and prevalence of inflammatory bowel disease among U.S. veterans: a national cohort study. Inflamm Bowel Dis. 2013 Apr;19(5):1059-64. doi: 10.1097/MIB.0b013e31828028ca. PMID: 23448789; PMCID: PMC3972034.
