LOUIS — Cancer patients have both an increased risk of developing atrial fibrillation (AF), and those with the condition have a higher risk of cardiovascular-related death at a year.
For most patients, the best solution is use of anticoagulant therapy. The problem for cancer patients, however, is that they are at increased risk of anticoagulant-related bleeding.
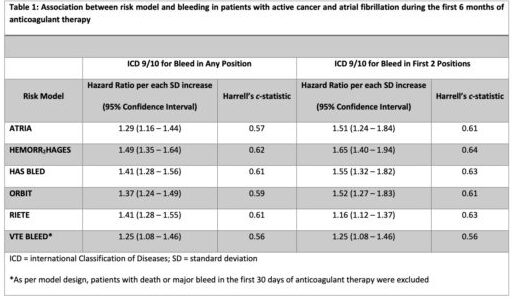
New research presented at the recent 64th Annual ASH Meeting and Exposition in New Orleans described how, while a range of risk prediction models are available to estimate the risk of anticoagulant-related bleeding in patients with AF, they mostly were developed in patients without cancer. “Thus, the utility of these models in cancer is unknown,” said the lead researchers from the St. Louis VAMC Research Service and Washington University in St. Louis School of Medicine.
As a result, the study team sought to evaluate the performance of existing bleeding risk models in a cohort of patients with active cancer and AF starting on anticoagulant therapy. To do that, they used a nationwide cohort of U.S. veterans, identifying patients with active cancer and AF with a new prescription for anticoagulant therapy between 2012 and 2018.
For purposes of the study, cancer was defined as active if the diagnosis of AF occurred within three months prior to up to six months after a new cancer diagnosis or in the setting of incurable hematological cancers (e.g., multiple myeloma, chronic myelogenous leukemia) or metastatic solid tumors.
Anticoagulant-related major bleeding (MB) within six months of anticoagulant initiation was identified inpatient ICD-9/10 codes.
With 5,716 patients meeting inclusion criteria into the cohort, the most common types of cancer identified included:
- hematological malignancies (24%),
- lung (20%),
- prostate (18%),
- non-prostate genitourinary (11%), and
- gastrointestinal (10%) cancer.
The mean age at the start of anticoagulant therapy was 73.4 years, with median overall survival of 41.1 months. Within the cohort, anticoagulant therapy received included warfarin in 2,040; LMWH in 179; warfarin, 179 fondaparinux, in 5; and direct-acting oral anticoagulants (DOAC) in 3,495.
Within six months of anticoagulant start, 5.7% of patients had a major bleeding event (MB); 7.7% had one within 12 months. The researchers reported that the most common type of bleeds within the first six months of anticoagulant therapy was gastrointestinal (47%), followed by hematuria (28%) and hemoptysis (13.7%). Intracranial hemorrhage occurred in 2.4% of bleeding events.
“In this cohort of 5,716 patients with active cancer and atrial fibrillation starting on anticoagulant therapy, available clinical risk models moderately predicted bleeding,” the authors pointed out. “The addition of cancer-specific risk factors might improve accuracy of the available models.”
- Sanfilippo KM, Luo S, Schoen MW, Chang S-H, Gage BF. Available Risk Scores Modestly Predict Hemorrhage in Patients with Atrial Fibrillation and Cancer. Presented at the 64th Annual ASH Meeting and Exposition; December 2022; New Orleans, LA