VA Researchers Also Can’t Identify Protective Effects
BIRMINGHAM, AL—Multiple observational studies have suggested that beta blockers benefit patients with moderate or severe chronic obstructive pulmonary disease and coexisting cardiovascular disease, with outcomes similar to those observed in patients without COPD.
A new article in the New England Journal of Medicine also points out that several nonrandomized observational studies involving COPD patients have found indications that beta blockers reduce the risk of exacerbations and death, regardless of the presence of cardiac disease, although that has not been confirmed in clinical trials. It is well established, however, that beta blockers reduce mortality in patients after myocardial infarction and in those with heart failure, according to the report.1
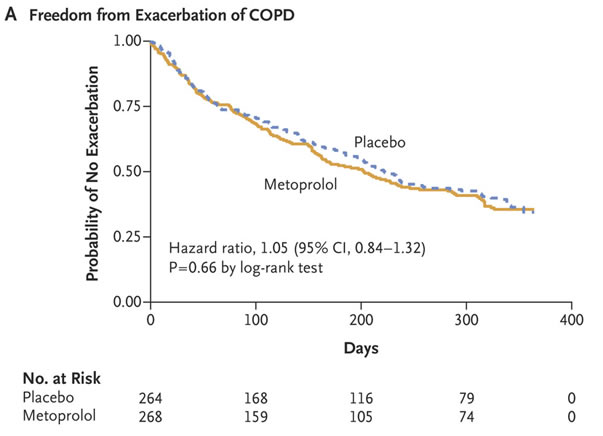
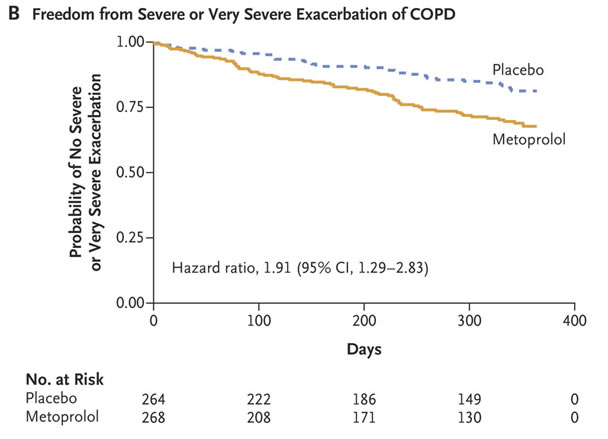 Yet, according to the BLOCK COPD Trial Group led by Birmingham, AL, VAMC and University of Alabama at Birmingham researchers, COPD patients “often are not treated with this class of medications, even when they have an evidence-based indication for the use of such drugs, because of concern about possible adverse effects on lung function.”
Yet, according to the BLOCK COPD Trial Group led by Birmingham, AL, VAMC and University of Alabama at Birmingham researchers, COPD patients “often are not treated with this class of medications, even when they have an evidence-based indication for the use of such drugs, because of concern about possible adverse effects on lung function.”
VAMCs in Minneapolis, MN; Ann Arbor, MI, Cincinnati, and the North Florida-South Georgia Veterans Health System in Gainesville, FL, also participated in the study, which was funded by the DoD.
To better inform the debate, the study team conducted a prospective, randomized trial, assigning COPD patients between the ages of 40 and 85 years to receive either a beta blocker (extended-release metoprolol) or placebo.
The 532 participants all had a clinical history of COPD, along with moderate airflow limitation and an increased risk of exacerbations, which was determined by a history of exacerbations during the previous year or the prescribed use of supplemental oxygen. The mean (±SD) age of the patients was 65.0±7.8 years, while the mean forced expiratory volume in 1 second (FEV1) was 41.1±16.3% of the predicted value. Excluded were patients who were already taking a beta blocker or who had an established indication for the use of such drugs.
Defined as the primary end point was the time until the first exacerbation of COPD during the treatment period, which, depending on the adjusted dose of metoprolol, ranged from 336 to 350 days. The authors report that the trial was stopped early because of futility with respect to the primary end point and safety concerns.
Continue Reading this Article: Higher Hospitalization Risk


metoprolol succinate cardio-selective, would be interesting to see this same study with carvedilol.