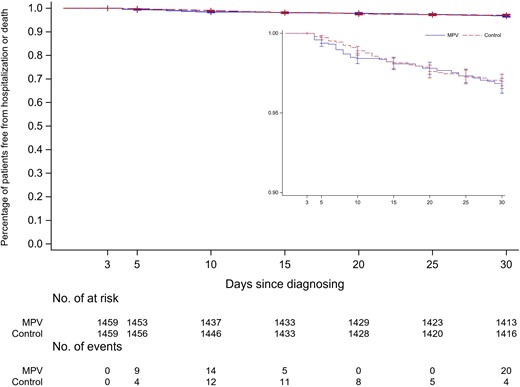
Click to Enlarge: Kaplan-Meier curves depicting percentage of patients without hospitalization or death among those treated with molnupiravir and untreated controls (inset: magnification of the graph to show details). Logrank P = .67. Abbreviation: MPV, molnupiravir. Source: The Journal of Infectious Disease
PITTSBURGH — Molnupiravir, an antiviral medication that is used to treat COVID-19, is not associated with significant clinical benefits, such as preventing hospitalizations or death in veterans with COVID-19, according to a recent study.
In the matched cohort study published in The Journal of Infectious Diseases, the researchers, including scientists at VA Pittsburgh Healthcare System, determined the rate of hospitalization or death within 30 days of COVID-19 diagnosis among patients treated with molnupiravir and untreated controls. The efficacy and clinical benefit of molnupiravir in treating COVID-19 have been unclear.1
In December 2021, molnupiravir and nirmatrelvir/ritonavir, marketed as Paxlovid, two novel oral antiviral agents, were granted emergency use authorization by the U.S. Food and Drug Administration for treating early symptomatic patients with mild to moderate COVID-19 who were at high risk of progression to severe disease. “Early clinical trials have demonstrated a benefit in clinical outcomes, but the effect has not been uniform or experienced by all demographic and clinical subgroups,” the study reported.
Participants in the study were non-hospitalized, previously uninfected veterans with a first confirmed SARS-CoV-2 infection between Jan. 1, 2022 and Aug. 31, 2022. They were either prescribed molnupiravir within three days of COVID-19 diagnosis or were matched individuals who were not prescribed molnupiravir. To qualify, participants couldn’t have experienced hospitalization or death within three days of testing positive.
Eligible individuals were those in the VA COVID-19 Shared Data Resource, which contains demographic, clinical, laboratory, vital status and episodes-of-care information on all veterans with a laboratory-confirmed diagnosis of SARS-CoV-2 infection and who received a SARS-CoV-2 vaccine within the VA. The database is updated regularly with information from multiple sources.
The study’s primary outcome measure was hospitalization or death within 30 days of the COVID-19 diagnosis date. The researchers allowed a three-day window from the time of COVID-19 diagnosis to be included in either group, the study reports.
“We did not find an overall significant benefit of molnupiravir in preventing hospitalizations or death in veterans with COVID-19 in the short term,” Adeel A. Butt, MBBS, professor of medicine and population health sciences at Weill Cornell Medical College in New York, NY, and a staff scientist at Pittsburgh, VA Pittsburgh Healthcare System, told U.S. Medicine. “However, there was a subgroup of veterans with COVID-19 who presented without any symptoms and experienced a reduction in hospitalization or 30-day mortality.”
The study found that, among 1,459 matched pairs of participants, the incidence of hospitalization or death was not different among participants treated with molnupiravir vs. untreated controls. In addition, no benefit was observed among patients older than 60 or those 60 years old and younger, those with specific comorbidities or by vaccination status. “A significant benefit was observed in asymptomatic but not in symptomatic persons,” according to the study.
Asymptomatic individuals who were prescribed molnupiravir “experienced a significant reduction in hospitalization or death.” The study suggests the “benefit from molnupiravir might be limited to patients with the mildest form of disease who are prescribed molnupiravir early in the course of the illness.”
The study confirmed previous studies that demonstrate a benefit of molnupiravir “only in a subgroup of infected individuals who are asymptomatic” at the presentation of illness, according to the report. Molnupiravir often has been used in patients who, often because of drug interactions, weren’t candidates to use Paxlovid.
When caring for populations with COVID-19, healthcare professionals should seek the advice of colleagues who have experience treating the illness, the researchers recommended.
“We believe that vaccination is still the most effective intervention in preventing severe consequences of COVID-19,” Butt wrote in an email. “Many other therapeutic agents are now available. Clinicians experienced in treating COVID-19 should be consulted for proper management of patients who present with moderately severe or severe illness.”
The study’s results are significant because they don’t reflect previous findings of randomized clinical trials on molnupiravir use.
No Significant Benefit
“In our study, we were not able to demonstrate a significant benefit of molnupiravir that was observed in the randomized clinical trials and some other observational studies,” Butt explained. “Randomized controlled trials are tightly regulated studies, which are critically important in determining the efficacy of new treatments. Our study demonstrates that the results from real-world studies can be different than those observed in clinical trials. Real-world studies are important since they more closely resemble the patients and settings where interventions are actually used. However, real-world studies, like ours, should be interpreted with caution due to some inherent design limitations. These results should be replicated in other studies and other settings to ensure their validity beyond a single population or setting.”
The study adds to growing evidence that molnupiravir may be effective “only in asymptomatic individuals or mild disease and when given early in the course of illness.” The researchers suggest that prescribing molnupiravir “only to those who may expect a benefit can prevent untoward adverse events and lower the costs of therapy.”
The results suggested that more studies are needed to understand molnupiravir.
“We should evaluate the role of molnupiravir more critically and through more studies before using it as a first line therapeutic agent for COVID-19,” Butt wrote.
- Butt AA, Yan P, S Shaikh O, B Omer S, B Mayr F, B Talisa V. Molnupiravir Use and 30-Day Hospitalizations or Death in Previously Uninfected Non-hospitalized High-risk Population with COVID-19. J Infect Dis. 2023 Jun 1:jiad195. doi: 10.1093/infdis/jiad195. Epub ahead of print. PMID: 37260359.
