Nirmatrelvir-Ritonavir Reduced Some Thromboembolic Events
SEATTLE — Nirmatrelvir-ritonavir, an oral antiviral drug used to treat COVID-19, only appears to reduce some post-COVID-19 conditions, such as combined thromboembolic events, according to a recent study.
The hope had been that use of the drug would help prevent a range of symptoms associated with so-called Long COVID.
The study published in the Annals of Internal Medicine measured how effective outpatient treatment of COVID-19 with nirmatrelvir-ritonavir was in preventing post-COVID-19 conditions.1
Infection with SARS-CoV-2, the virus that causes COVID-19, is “believed to increase the risk of several medical conditions long after acute illness, and post-COVID-19 conditions, which involve multiple organ systems, include pulmonary, cardiovascular, cerebrovascular, thromboembolic, neurocognitive, mental health, metabolic, renal and gastrointestinal disorders,” the study authors explained.
A national Centers for Disease Control and Prevention (CDC) study suggested that “one in five COVID-19 survivors aged 18 to 64 years old and one in four survivors aged 65 years or older experienced a condition that could potentially be attributed to previous COVID-19 illness,” the study reported. “Ritonavir-boosted nirmatrelvir (nirmatrelvir-ritonavir), among several other antiviral drugs, is recommended as a preferred antiviral treatment for COVID-19, and in many settings, nirmatrelvir-ritonavir has been the most prescribed treatment agent.”.
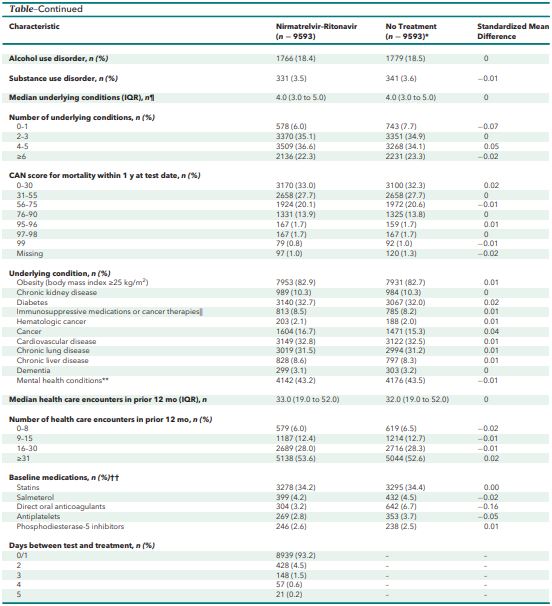
Click to Enlarge: CAN = Care Assessment Need; NIH = National Institutes of Health.
* Baseline characteristics represent equally weighted matched untreated comparators (up to 4 untreated matched comparators).
† Based on rural-urban commuting area codes.
‡ Regions are based on Veterans Integrated Service Networks (VISNs). West includes VISNs 19 to 22; Midwest includes VISNs 10, 12, 15, and 23; Northeast includes VISNs 1, 2, 4, and 5; and South includes VISNs 6 to 9, 16, and 17.
§ Any of 15 prespecified COVID-19–related symptoms present on the day of the SARS-CoV-2 test with a positive result or within the preceding 30 days.
‖ See the Supplement.
¶ Medical conditions associated with severe COVID-19 risk according to the Centers for Disease Control and Prevention (Supplement Table 2).
** Includes major depressive disorder, bipolar disorder, posttraumatic stress disorder, and schizophrenia.
†† Baseline medications prescribed in the 90 days before the test-positive date with clinically significant drug–drug interactions with nirmatrelvir–ritonavir: statins (simvastatin, lovastatin, atorvastatin, rosuvastatin), salmeterol, direct oral anticoagulants (apixaban, rivaroxaban), antiplatelets (clopidogrel, ticagrelor), and phosphodiesterase-5 inhibitors (sildenafil, tadalafil, vardenafil). Source: American College of Physicians
Studies are needed to “evaluate whether COVID-19 drug therapies, including nirmatrelvir-ritonavir, are effective at reducing risk for post–COVID-19 conditions,” the study authors suggested. “Whether acute treatment with nirmatrelvir-ritonavir reduces the risk of post-COVID-19 conditions remains one of the most important unanswered questions related to COVID-19 and has not been addressed by randomized controlled trials.”
In this study, the authors investigated whether acute treatment of COVID-19 with nirmatrelvir-ritonavir (Paxlovid) reduced the risk of development of a number of post-COVID-19 conditions up to six months after the infection. They conducted a target trial emulation using the national electronic health records of the VHA for veterans who tested positive for SARS-CoV-2, the authors explained.
Participants in the study, who were identified retrospectively, were nonhospitalized veterans in VHA care who were at risk for severe COVID-19 and tested positive for SARS-CoV-2 from Jan. 1, 2022, through July 31, 2022. The study compared matched cohorts receiving nirmatrelvir-ritonavir versus no treatment. Participants were excluded who received any COVID-19 treatment before the test-positive date and did not have at least one risk factor for progression to severe COVID-19. Of the participants, 86% were male, the median age was 66 years old, and 17.5% were unvaccinated, the authors pointed out.
The research team investigated “COVID-19 treatment in VHA during the Omicron variant era and evaluated the incidence of post-COVID-19 conditions 31 to 180 days after treatment initiation,” the study added.
The study authors were affiliated with VA Puget Sound Health Care System in Seattle; VA Connecticut Healthcare System in West Haven, CT; VA Portland, OR, Healthcare; the VA Center for Medication Safety-Pharmacy Benefit Management Services in Hines, IL; VA Ann Arbor, MI, Healthcare System; the Durham, NC, VAMC; the VA Palo Alto, CA, Healthcare System; and the Office of Research and Development in Washington, DC.
“The most important finding was that, out of 31 potential post-COVID-19 conditions that we evaluated, only combined thromboembolic events (venous thromboembolism and pulmonary embolism) seemed to be reduced by nirmatrelvir-ritonavir (Paxlovid),” George Ioannou, BMBCh, MS., director of hepatology at the VA Puget Sound Health Care System told U.S. Medicine.
Preventing Post-COVID-19 Conditions
“I think our results are important as investigators around the world are trying to understand how to best prevent (or treat) post-COVID-19 conditions, which are of great concern to the public. I hope our results will be interpreted in the context of other excellent studies that have been published,” Ioannou said.
The results suggest that “considerations about post-COVID-19 conditions may not be an important factor in COVID-19 treatment decisions,” the study concluded.
Nirmatrelvir-ritonavir has been consistently shown in randomized controlled trials and in observational studies, including this study, “to reduce the rate of death and hospitalization related to acute infection in high-risk patients,” Ioannou wrote.
“Therefore, such high-risk patients should consider treatment with nirmatrelvir-ritonavir as recommended by the CDC,” Ioannou said.
One limitation of the study was that post-COVID-19 conditions that were determined “using International Classification of Diseases, 10th Revision, codes may be inaccurate,” the study authors suggested. “Evaluation of many outcomes could have resulted in false associations with combined thromboembolic events by chance,” the authors continued.
Also, using electronic health record data, the researchers couldn’t determine “symptom burden or date of symptom onset accurately, and patients treated with nirmatrelvir-ritonavir might have been more likely to be symptomatic on their treatment date than their matched untreated comparators on their assigned paired index date,” according to the authors.
In addition, determining “outpatient COVID-19 treatments or incident post-COVID-19 conditions may be incomplete despite integrating multiple data sources (VA electronic health records, Community Care, and Centers for Medicare & Medicaid Services) and limiting the study population to veterans with a recent primary care visit who were more likely to seek care within the VHA,” the authors explained.
- Ioannou GN, Berry K, Rajeevan N, Li Y, et. al. Effectiveness of Nirmatrelvir-Ritonavir Against the Development of Post-COVID-19 Conditions Among U.S. Veterans : A Target Trial Emulation. Ann Intern Med. 2023 Nov;176(11):1486-1497. doi: 10.7326/M23-1394. Epub 2023 Oct 31. PMID: 37903369; PMCID: PMC10620954.