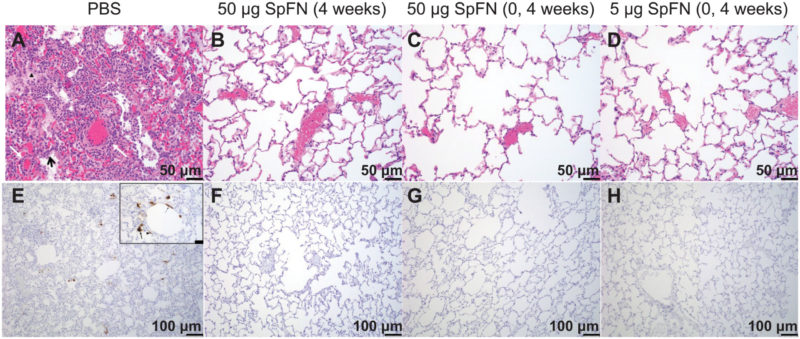
Click To Enlarge: Histopathology and virus detection in the lungs of SpFN-vaccinated and unvaccinated control rhesus macaques after SARS-CoV-2 respiratory challenge.
(A to D) Paraffin-embedded lung parenchymal tissue sections collected at 7 days after challenge were stained with hematoxylin and eosin (H&E). (E to H) Samples were also used for IHC to measure viral antigen (brown). Representative images at two different magnifications, one for H&E and the other for IHC, are presented. The symbols (A) show inflammatory debris (white star), type II pneumocyte hyperplasia (black arrow), edema (black triangle), and vasculitis of small- to medium-caliber blood vessels (white arrows). Viral antigen is seen as brown aggregates with an inset at higher magnification (scale bar, 20 μm) showing viral antigen was detected in alveolar pneumocytes (thick arrow), pulmonary macrophages (arrowhead), and, rarely, endothelial cells (thin arrow) (E).
SILVER SPRING, MD — Based on recent preclinical study results, the Spike Ferritin Nanoparticle (SpFN) COVID-19 vaccine is showing significant promise. Not only does it elicit a potent immune response, according to Army researchers, it also appears to provide broad protection against SARS-CoV-2 variants of concern as well as other coronaviruses.
“The recent success in the rapid development of safe and efficacious SARS-CoV-2 vaccines has been tempered by the emergence of virus variants to which vaccine-induced immunity has shown diminished potency or efficacy. Thus there remains a need for next-generation vaccines that target the broadening antigenic diversity of SARS-CoV-2 and related coronaviruses,” wrote Walter Reed Army Institute of Research (WRAIR) researchers, who developed the SpFN. It is on a protein subunit nanoparticle vaccine platform, which means it presents a fragment of a virus to the immune system to elicit a protective response.
Although results of early human trials of the human SpFN vaccine are not yet available, a series of recently published preclinical studies—including a study in nonhuman primates published Dec. 16 in Science Translational Medicine—showed that the vaccine not only elicits a potent immune response but may also provide broad protection against SARS-CoV-2 variants of concern as well as other coronaviruses.1
“The accelerating emergence of human coronaviruses throughout the past two decades and the rise of SARS-CoV-2 variants, including most recently omicron, underscore the continued need for next-generation preemptive vaccines that confer broad protection against coronavirus diseases,” Kayvon Modjarrad, MD, PhD, director of the Emerging Infectious Diseases Branch at WRAIR, co-inventor of the vaccine and the U.S. Army lead for SpFN, explained in an EIDB press release. “Our strategy has been to develop a ‘pan-coronavirus’ vaccine technology that could potentially offer safe, effective and durable protection against multiple coronavirus strains and species.”
The major vaccines that have progressed to human efficacy trials and have been granted either approval or emergency use authorization all present the SARS-CoV-2 spike protein that is based on the genetic sequence of the Wuhan-Hu-1 isolate, one of the first available COVID-19 genomes. All of these vaccines have demonstrated protective efficacy in nonhuman primates against respiratory mucosal challenge with the closely matched USA-WA1/2020, the authors wrote in the journal. “These earlier animal studies, however, did not evaluate the neutralization capacity of serum against other coronavirus species.”
In the recent study of 32 male and female Chinese-origin rhesus macaques—which are genetically and anatomically similar to humans—WRAIR researchers demonstrated that SpFN elicited high titers of antibodies that neutralized SARS-CoV-2 and rapidly protected against SARS-CoV-2 infection. “SARS-CoV-2 vaccine efficacy studies in nonhuman primates generally compare elicited antibody responses to those from patients who have recovered from COVID-19,” the authors wrote. They found that the neutralizing activity in the animals receiving two 50-microgram doses of SpFN was 10-fold higher than that in recovering patients.
The researchers also found that, compared to wild type virus, SpFN elicited serum virus neutralizing activity that was either higher or equivalent against four major variants of concern variants of concern. SpFN also induced robust neutralizing activity against SARS-CoV-1. The virus responsible for the SARS outbreak in 2002–2003, SARS-CoV-1 is a separate species that has 26% and 36% sequence divergence in the spike protein and S1 subunit, respectively, which is important for protection in animal models, the authors noted.
The authors said the potent neutralizing antibody responses the researchers observed may offer advantages for both vaccine efficacy and durability. “Thus far, neutralizing activity has been predictive of efficacy in human trials, because vaccines that generate lower antibody titers have diminished efficacy,” they wrote, adding that the length of immunity conferred by current SARS-CoV-2 vaccines is unknown. “Among viral infections for which neutralizing antibodies are the primary correlate of protection, peak titers have been shown to be predictive of durability and may serve as one of several indicators of the length of vaccine-elicited protective immunity. As such, SpFN may offer some measure of a durable immune response, although this will require empirical confirmation.”
SpFN entered Phase 1 human trials in April 2021, according to a WRAIR press release. Early analyses of those trial results will provide insights into whether SpFN’s potency and breadth, as demonstrated in preclinical trials, will carry over into humans. The data will also allow researchers to compare SpFN’s immune profile to that of other COVID-19 vaccines already authorized for emergency use.
“This vaccine stands out in the COVID-19 vaccine landscape,” Modjarrad said. “The repetitive and ordered display of the coronavirus spike protein on a multi-faced nanoparticle may stimulate immunity in such a way as to translate into significantly broader protection.”
WRAIR developed a secondary candidate vaccine, a SARS-CoV-2 Spike Receptor-Binding Domain Ferritin Nanoparticle (RFN) vaccine, which targets a smaller part of the coronavirus Spike protein than the SpFN vaccine. Results from a study, published recently in the Proceedings of the National Academy of Sciences, show that this vaccine potentially offers similar protection against an array of SARS-CoV-2 variants and SARS-CoV-1.
“The threat from COVID-19 continues as it evolves, and eventually there will be other emerging disease threats,” said Col. Nelson Michael, MD, PhD, director of the Center for Infectious Diseases Research at WRAIR. “Our investment in developing a next generation vaccine is an important step toward getting ahead of COVID-19 and future disease threats.”
- Jones MG, King D, Ahmed A, et al. A SARS-CoV-2 ferritin nanoparticle vaccine elicits protective immune responses in nonhuman primates. Science Translational Medicine. December 16, 2021. DOI: 10.1126/scitranslmed.abi5735
