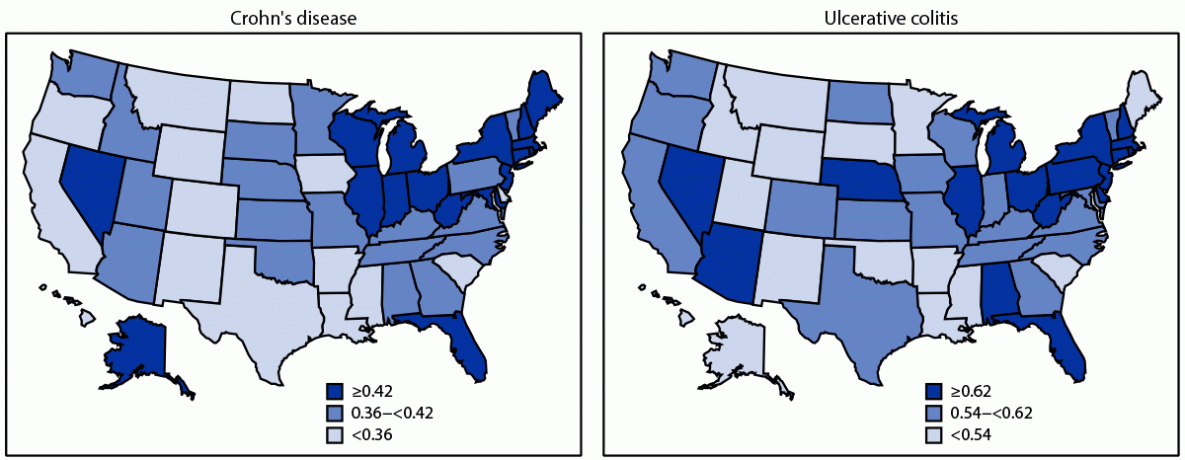
Click to Enlarge: * Age-adjusted to the 2000 U.S. Census population aged ≥67 years based on three age groups (67–74, 75–84, and ≥85 years). Census Data
† State-level age-adjusted prevalence estimate (%) was categorized into tertiles. Source Center for Disease Control
CHICAGO — A few months ago, the Food and Drug Administration approved the first JAK Inhibitor for adults with moderately to severely active Crohn’s disease who have had an inadequate response or intolerance to one or more tumor necrosis factor blockers.
Upadacitinib, marketed as Rinvoq, already has indications for ulcerative colitis, as do several others in the Janus Kinase inhibitors (JAK) inhibitor class, including tofacitinib, a nonselective small molecule JAK inhibitor, and filgotinib, another selective JAK-1 inhibitor approved for moderate-to-severe active ulcerative colitis. Ulcerative colitis (UC) and Crohn’s disease (CD) are the most common types of inflammatory bowel disease (IBD), which affects about 66,000 veterans, according to the Crohn’s and Colitis Foundation.
A review article published in the journal Expert Review of Clinical Immunology evaluated the current status of JAK inhibitors to treat ulcerative colitis and Crohn’s disease. The research team included researchers from the Loma Linda, CA, VA Loma Linda Healthcare System.1
“JAK inhibitors are the newest addition to a gastroenterologist’s armamentarium to treat moderate-severe ulcerative colitis, and with the recent FDA approval of upadacitinib, also for the treatment of moderate-severe Crohn’s disease,” Stephen B. Hanauer, MD, professor of medicine at Northwestern University Feinberg School of Medicine, told U.S. Medicine. “It is important to understand the risks and benefits and utilization of this mechanism of action applied to IBD [inflammatory bowel disease] as JAKs are also approved for other indications such as rheumatoid arthritis, psoriatic arthritis and atopic dermatitis, at different doses than used for IBD.”
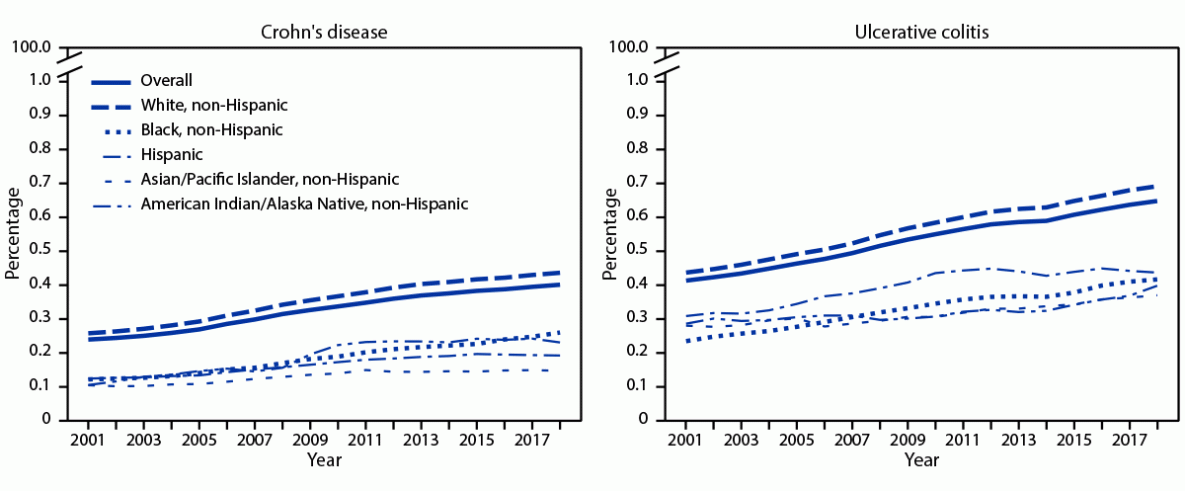
Click to Enlarge: Abbreviation: APC = annual percentage change.
* Age-adjusted to the 2000 U.S. Census population aged ≥67 years based on three age groups (67–74, 75–84, and ≥85 years). Census Data
† Trends in age-adjusted prevalence estimates were assessed in linear regressions weighted with the estimates-associated inversed standard errors. The estimated prevalence was natural logarithm transformed. For Crohn’s disease, APC = 3.4% for overall, 3.5% for non-Hispanic White persons, 5.0% for non-Hispanic Black persons, 3.2% for Hispanic persons, 2.7% for Asian/Pacific Islander persons, and 5.3% for American Indian/Alaska Native persons. For ulcerative colitis, APC = 2.8% for overall, 2.9% for non-Hispanic White persons, 3.5% for non-Hispanic Black persons, 2.5% for Hispanic persons, 1.8% for Asian/Pacific Islander persons, and 1.5% for American Indian/Alaska Native persons. All were statistically significant (p<0.001).
§ The conversion from the International Classification of Diseases, Ninth Revision diagnosis codes to the International Classification of Diseases, Tenth Revision diagnosis codes occurred on October 1, 2015. Source Center for Disease Control
The researchers concluded that JAK inhibitors are a “safe and effective once or twice daily oral therapy for moderate-severe ulcerative colitis and Crohn’s disease.”
“JAK inhibitors are very efficacious and work very quickly, such that they improve symptoms of ulcerative colitis or Crohn’s disease within days and are currently being studied in patients with severe ulcerative colitis. They are generally quite safe but in rheumatoid arthritis, not in IBD, are associated with a greater risk of cardiovascular or thromboembolic events than tumor necrosis blockers in patients over the age of 50 with cardiovascular risk factors,” Hanauer explained.
The review found “there are no differences in treating with JAK inhibitors across adult patients of different ethnicities, races or ages, although elderly patients will have a greater risk of cardiovascular and thromboembolic events as well as malignancies compared with younger patients,” Hanauer wrote in an email.
For healthcare professionals caring for patients with IBD, the researchers suggest that “JAK inhibitors provide safe and effective, once or twice daily oral therapies that are well-tolerated and work both quickly and on a long-term basis.” There is “strong evidence for clinical and endoscopic remissions (and histological healing in ulcerative colitis) for both patients who are naive to biologics or have had inadequate responses to biologic agents. The U.S. Food and Drug Administration (FDA) recommends this treatment for patients with inadequate responses to biologics in the United States, although there are no such recommendations in Europe,” Hanauer said.
“JAK inhibitors are considered among the ‘advanced therapies’ for IBD and are approved for the treatment of moderate to severe ulcerative colitis in adults with pending approvals for Crohn’s disease in the U.S. They offer non-immunogenic, oral options for patients not responding to other conventional agents, but they have been restricted by the FDA to patients with inadequate response to TNF blockers,” the review article reported.
Alternative to Biologic Agents
The review explained that JAK inhibitors “offer rapidly acting oral alternatives to biologic agents for moderate-severe ulcerative colitis, and the risks of cardiovascular and thrombotic events noted in rheumatoid arthritis have not been observed in IBD clinical trials. However, monitoring of infections (primarily herpes zoster) and risk factors for cardiovascular and thrombotic complications is appropriate.”
Of special interest to the VA, considering its patient population, was an article in the Morbidity & Mortality Weekly Report finding increased prevalence of IBD among older adults. The report in the national Centers for Disease Control and Prevention (CDC) publication noted that IBD prevalence generally is “higher among non-Hispanic White persons than it is among persons in other racial/ethnic groups.”
In 2018, the CDC researchers reported that 0.40% and 0.64% of 25.1 million Medicare fee-for-service beneficiaries older than 67 had received a diagnosis of Crohn’s disease or ulcerative colitis. “From 2001 to 2018, the age-adjusted prevalence of IBD increased among all racial/ethnic groups; the highest annual percentage increase was among non-Hispanic Black persons,” they pointed out.
The CDC suggested that study findings “of increasing prevalence among older adults across all racial/ethnic groups, especially the higher rate of increase among certain racial and ethnic minority groups, underscore the importance for promoting health equity, guiding efforts to tailor disease management strategies for different populations, and continuing to monitor the temporal trends of the disease.”
Increases in prevalence are notable, the study added, with the number of affected patients worldwide increasing from 3.7 million in 1990 to 6.8 million in 2017
- Gupta N, Papasotiriou S, Hanauer S. The evolving role of JAK inhibitors in the treatment of inflammatory bowel disease. Expert Rev Clin Immunol. 2023 Jul-Dec;19(9):1075-1089. doi: 10.1080/1744666X.2023.2214728. Epub 2023 May 24. PMID: 37226522.
- Xu F, Carlson SA, Liu Y, Greenlund KJ. Prevalence of Inflammatory Bowel Disease Among Medicare Fee-For-Service Beneficiaries—United States, 2001-2018. MMWR Morb Mortal Wkly Rep 2021;70:698-701. DOI: http://dx.doi.org/10.15585/mmwr.mm7019a2external icon
