BALTIMORE — Diabetes patients who are treated with anti-vascular endothelial growth factor (VEGF) injections, the primary treatment for diabetic retinopathy, have a higher likelihood of experiencing adverse events, such as acute myocardial infarction, cardiovascular disease or kidney disease, according to a recent study.
The retrospective, longitudinal study published in JAMA Ophthalmology investigated the safety of anti-VEGF agents, particularly in high-risk diabetes patients. Anti-VEGF agents have been an effective treatment for diabetic retinopathy, but safety data have been inconclusive, the study reports.1
Researchers for the study were affiliated with the Michael E. DeBakey VA Medical Center in Houston.
Diabetes is increasing in prevalence, and an estimated 30.3 million people or 9.4% of the United States population had diabetes in 2015. Ocular complications from diabetic retinopathy, an eye condition that can cause vision loss and blindness in people who have diabetes, and diabetic macular edema are leading causes of vision loss among working-aged adults, according to the study.
Diabetic retinopathy is managed by administering anti-VEGF agents. The study reported that concerns have been raised about the systemic safety of anti-VEGF agents in some high-risk patients.
The study used information from the Corporate Data Warehouse, a large database of patients in the VHA that includes data on the physical and mental health of veterans. Patients in the study were adults with Type 2 diabetes who had been seen at any VA healthcare facility in the U.S. between Jan. 1, 2011, and Dec. 31, 2012. Included were 1.7 million patients with a mean age of 63.8 years old; diabetic retinopathy was present in 476,013 patients (27.5%), and 14,022 patients (0.8%) received anti-VEGF injections.
Data were obtained on systemic adverse events among the patients from Jan. 1, 2013, to Dec. 31, 2017. All patients with diabetes, regardless of whether they received anti-VEGF injections, were included. Excluded were patients with a history of prior systemic adverse events and those who received an intravitreal anti-VEGF injection between Jan. 1, 2011, and Dec. 31, 2012. Data were analyzed from October 2019 to March 2023, according to the study.
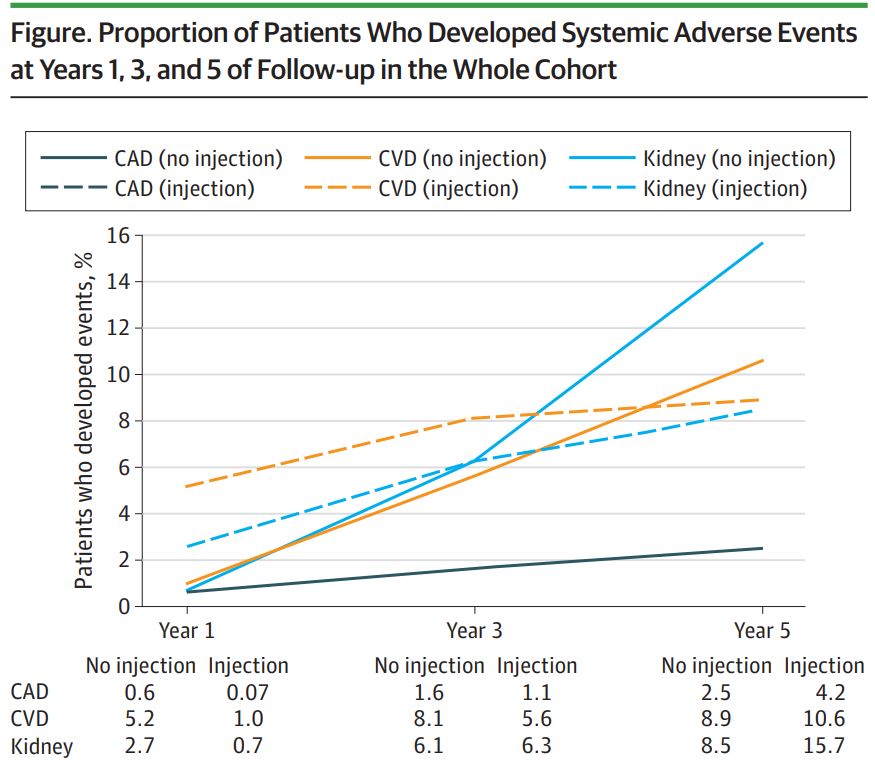
“Intravitreal anti-VEGF injections were not only associated with increased risk of any systemic adverse event overall, but also with increased risk of individual systemic adverse events including CAD, cerebrovascular disease and kidney disease,” study author Sidra Zafar, MD, third-year resident in the ophthalmology residency program at Wilmer Eye Institute at Johns Hopkins Medicine, told U.S. Medicine.
Who’s at Most Risk
“Our study found that patients who were older, male and of Hispanic/Latino ethnicity were at increased risk of developing systemic adverse events,” Zafar wrote in an email.
Among the patients with Type 2 diabetes, the researchers found that 321,940 (18.6%) developed systemic adverse events between 2013 and 2017. The five-year cumulative incidence of any systemic adverse event was 37.0% in the anti-VEGF injection group versus 18.4% in the noninjection group, according to the study.
“Anti-VEGF injections were independently associated with a higher likelihood of developing any systemic adverse event when controlling for age, race, sex, ethnicity, tobacco use, severity of diabetic retinopathy, Deyo-Charlson Comorbidity Index score, mean hemoglobin A1c, total number of injections and statin use,” the study reported.
“The findings indicate that continued or prolonged anti-VEGF use may increase the risk of systemic adverse events in patients with diabetes,” the researchers added.
“Despite their effectiveness for treating certain retinal diseases, intravitreal anti-VEGF injections do come with some risks,” Zafar explained. “Particularly, in high-risk patient population groups, such as those with diabetes, chronic and continued exposure to these medications can have systemic implications. However, it’s also important that we were unable to control for the duration of diabetes as a risk factor in our study, and it’s difficult to delineate how much of this is natural progression of disease versus anti-VEGF therapy contributing to the increased risk. That being said, it is still important to remember that these intravitreal agents may not be completely harmless from a systemic adverse event standpoint.”
Intravitreal anti-VEGF agents are currently the first-line therapy for diabetic eye disease. “It’s important that, when healthcare professionals talk to patients about the risks and benefits of intravitreal anti-VEGF injections, the systemic risks are discussed in more detail,” Zafar noted.
“I think this study raises some important questions about the systemic safety of these anti-VEGF injections, and as additional studies are designed, hopefully, we will move toward a consensus on systemic adverse effects of intravitreal anti-VEGF use,” Zafar said.
Because the study population consisted of mostly non-Hispanic white male patients, the typical population at the VA, future studies “should include more diverse cohorts to determine the generalizability of the findings,” the study suggested. One downside was that patients in the study received a combination of drugs, and the researchers were unable “to determine which anti-VEGF agents may be implicated in the observed associations,” the authors added.
The researchers recommended that “alternative therapies, such as intravitreal steroids or laser, may be an option for patients with diabetes who are at high risk of systemic adverse events.”
- Zafar S, Walder A, Virani S, Biggerstaff K, Orengo-Nania S, Chang J, Channa R. Systemic Adverse Events Among Patients With Diabetes Treated With Intravitreal Anti-Vascular Endothelial Growth Factor Injections. JAMA Ophthalmol. 2023 Jun 1:e232098. doi: 10.1001/jamaophthalmol.2023.2098. Epub ahead of print. PMID: 37261816; PMCID: PMC10236327.