Study Finds Beneficial AID Use Beyond Type 1 Diabetes
ROCHESTER, MN — Automated insulin delivery (AID) outperformed continuous glucose monitoring alone in Type 2 diabetes patients treated with insulin, according to a new study.
The research, which involved 319 patients at 21 sites in the United States and Canada, including a VA hospital, supported the use of the devices in the management of insulin-treated Type 2 diabetes, not only in Type 1 diabetes.
The Mayo Clinic-led 13-week, multicenter trial included adults with insulin-treated Type 2 diabetes who were randomly assigned in a 2:1 ratio to receive AID or to continue their pretrial insulin-delivery method (control group). Both groups received continuous glucose monitoring (CGM). Researchers from the Baltimore VAMC participated in the study, among others.
The primary outcome of the study, published in the New England Journal of Medicine, was the glycated hemoglobin level at 13 weeks.1
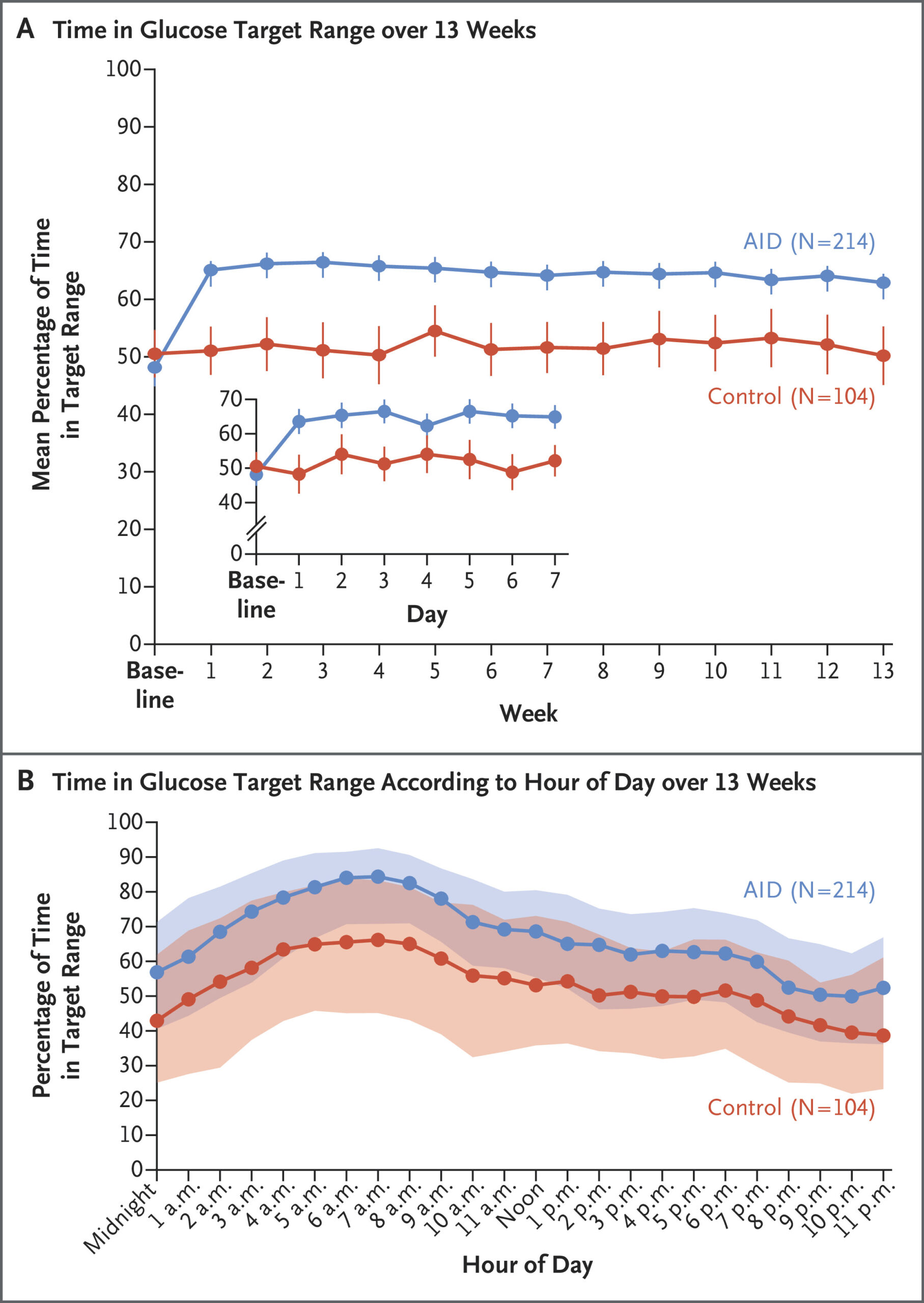
Results indicated that glycated hemoglobin levels decreased by 0.9 percentage points (from 8.2±1.4% at baseline to 7.3±0.9% at week 13) in the AID group and by 0.3 percentage points (from 8.1±1.2% to 7.7±1.1%) in the control group (mean adjusted difference, -0.6 percentage points; 95% confidence interval [CI], -0.8 to -0.4; P<0.001).
“The mean percentage of time that patients were in the target glucose range of 70 to 180 mg per deciliter increased from 48±24% to 64±16% in the AID group and from 51±21% to 52±21% in the control group (mean difference, 14 percentage points; 95% CI, 11 to 17; P<0.001),” the authors wrote. “All other multiplicity-controlled CGM outcomes reflective of hyperglycemia that were measured were significantly better in the AID group than in the control group.”
They added that the frequency of CGM-measured hypoglycemia was low in both groups, although a severe hypoglycemia event occurred in one patient in the AID group in the industry-funded trial.
Background information in the article noted that, while the benefits of AID systems are well established in patients with Type 1 diabetes, the technology’s efficacy and safety had not been established in those with Type 2 diabetes. The researchers pointed out that, even though promising results of AID in Type 2 diabetes have been reported, “patients have been evaluated either in uncontrolled trials or in short crossover trials with small sample sizes.”
The Randomized Trial Evaluating the Efficacy and Safety of Control-IQ+ Technology in Adults with Type 2 Diabetes Using Basal-Bolus Insulin Therapy (2IQP) sought to evaluate the efficacy and safety of AID in adults with Type 2 diabetes who were receiving multiple daily injections of insulin or using an insulin pump.
The study team added, “Treatment with medications such as glucagon-like peptide 1 (GLP-1) receptor agonists and sodium–glucose cotransporter 2 (SGLT2) inhibitors has enabled more patients with type 2 diabetes to have glycated hemoglobin levels below the American Diabetes Association target of 7%. Even so, a substantial proportion of those with type 2 diabetes who have elevated glycated hemoglobin levels may benefit from using an AID system.”
“Many patients with type 2 diabetes are being treated with GLP-1 receptor agonists or other noninsulin glucose-lowering medications,” according to the study. “Our trial results indicate that AID can further reduce glycated hemoglobin levels when added to a diabetes management regimen that includes one or more of these medications.”
The researchers advised that, in addition to a greater reduction in glycated hemoglobin levels in patients using AID, a similar benefit was report in CGM-measured hyperglycemia and related outcomes in a control group who used real-time CGM and continued their pretrial insulin-delivery method.
“Results appeared to be robust across a range of per-protocol and sensitivity analyses,” the authors explained. “The frequency of GAD [glutamic acid decarboxylase] antibodies (in 8% of the trial patients, although only 4% had a GAD level of more than 250 IU per milliliter) was not surprising, given that some patients with type 2 diabetes may be misdiagnosed and have a form of type 1 diabetes or features of both type 2 and type 1 diabetes. Results were unchanged when the patients with GAD antibodies were excluded from the analyses.”
Similar Benefit to Type 1 Diabetes
One of the findings was that the beneficial effect of AID in an insulin-treated Type 2 diabetes cohort was similar to the benefit observed in randomized, controlled trials involving adults with Type 1 diabetes. The participants represented a wide age range from 19 to 87 years.
Most of the trial participants opted to use a simple fixed-bolus regimen that allowed for minor adjustments for meal carbohydrate content. “Consequently, the results of this trial suggest that previous experience with an insulin pump or in-depth training in carbohydrate-counting methods are not prerequisites to the successful and safe use of AID to improve glycemia in patients with type 2 diabetes,” the authors noted. “Although we do not know the mechanism for the slight weight gain that was observed in the context of a reduction in total daily insulin delivery, possible explanations include decreased glycosuria, especially among the patients who had higher baseline glycated hemoglobin levels or increased calorie intake.”
The study team suggested that one significant aspect of their study was that it showed “the reduction in glycated hemoglobin levels that occurs with AID over and above the use of CGM without AID.”
Although only 4% of the patients were using an insulin pump before the trial, 71% were using CGM, which is a percentage that could be higher than what would be expected in an insulin-treated population with Type 2 diabetes.
- Kudva YC, Raghinaru D, Lum JW, Graham TE, et. Al.; 2IQP Study Group. A Randomized Trial of Automated Insulin Delivery in Type 2 Diabetes. N Engl J Med. 2025 Mar 19. doi: 10.1056/NEJMoa2415948. Epub ahead of print. PMID: 40105270.