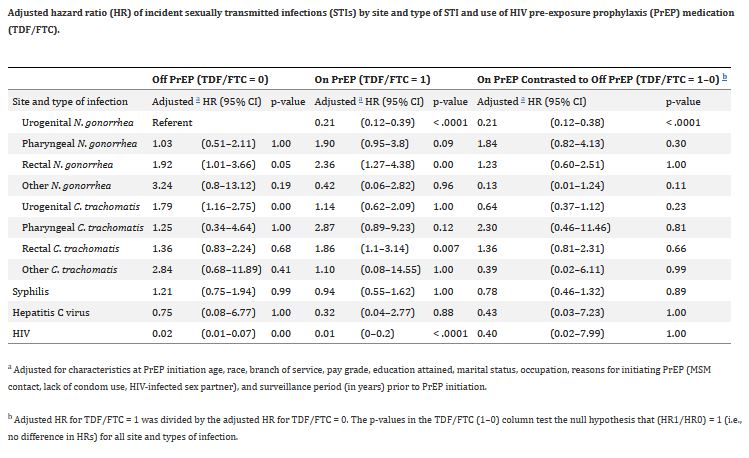
Click to Enlarge: a) Adjusted for characteristics at PrEP initiation age, race, branch of service, pay grade, education attained, marital status, occupation, reasons for initiating PrEP (MSM contact, lack of condom use, HIV-infected sex partner), and surveillance period (in years) prior to PrEP initiation.
b) Adjusted HR for TDF/FTC = 1 was divided by the adjusted HR for TDF/FTC = 0. The p-values in the TDF/FTC (1–0) column test the null hypothesis that (HR1/HR0) = 1 (i.e., no difference in HRs) for all site and types of infection. Source: PLoS One
BETHESDA, MD — Consistent use of HIV preexposure prophylaxis (PrEP) has been shown to reduce the risk of sexual risk of HIV acquisition by 99% among men who have sex with men (MSM). But, there has been concern that protection from HIV might lead to an increased risk of risky behaviors and the number of sex partners, putting men at greater risk for other sexually transmitted infections (STIs).
A new study of military servicemembers, however, suggested that the accessibility of PrEP might actually reduce the risk of other STIs indirectly and underscores the importance of counseling by physicians prescribing PrEP.
Concerns about the increased STI risk among men receiving PrEP arose in the years following the 2012 Food and Drug Administration (FDA) approval of tenofovir disoproxil fumarate/emtricitabine (TDF/TTC) for HIV prevention. Although HIV incidence in the U.S. decreased by 8% from 2015 to 2019—when TDF/TTC prescriptions were increasing—some research showed reportable sexually transmitted infections (STIs) such as chlamydia, gonorrhea and syphilis, increased by 30%.
While several studies have noted a rise in sexual behaviors that increase the risk of STIs following PrEP initiation, reported changes in STI incidence rates and prevalence proportions in MSM PrEP populations have been variable, reported authors of the new study, which was published in PLoS One. The new study was the first to assess the impact of PrEP initiation on STI incidence in the MHS.1
To assess whether the incidence of STIs increased among military servicemembers who were prescribed HIV PrEP soon after it received FDA approval, the researchers used electronic MHS pharmacy dispensation records to identify service members without HIV infection who were prescribed the medication TDF/FTC from Feb. 1, 2014, through June 10, 2016.
Healthcare providers reviewed clinical intake notes in electronic health records to verify initiation of PrEP and extracted reasons for initiation such as MSM contact, lack of condom use and whether sex partners were infected with HIV. For this analysis, the study team independently reviewed electronic health records and extracted all available positive laboratory results prior to PrEP initiation for incident Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), HIV, hepatitis C virus (HCV) and syphilis. Any CT or NG diagnosis less than 30 days following PrEP initiation was considered to have occurred before starting PrEP. An incident STI infection was defined as any new positive laboratory result for CT and NG, syphilis, HCV and HIV.
PrEP Lowered STI Rate
Among 755 male servicemembers in the final analysis, 477 (63%) were diagnosed with incident STIs, the study found. After adjustment for the effect of PrEP on site and type of infection, sociodemographic characteristics and reasons for starting PrEP, overall STI risk decreased significantly while on PrEP among male servicemembers who initiated PrEP in the MHS from Feb. 1, 2014, to June 10, 2016.
When stratifying for anatomical site and type of infection, however, the risk of extragenital gonorrhea infection and extragenital CT infection was greater on PrEP compared to off PrEP, although these values did not reach statistical significance, the researchers reported.
The authors speculated that the increase could be attributed to more frequent STI screening in the setting of PrEP adherence and follow-up care when following CDC PrEP guidelines than off PrEP, thus allowing for more opportunities to diagnose asymptomatic infections. Additionally, testing or screening of extragenital sites is more likely to occur, leading to higher rates of diagnosis of pharyngeal/rectal CT and/or NG.
“Moreover, in this analysis, more than half of servicemembers were cared for by dedicated sub-specialists with experience in HIV and STI care, and thus those engaged in PrEP services also receive more STI prevention counseling, potentially contributing to decreases in STI risk while on PrEP,” they wrote. “These data also suggest service members who were at high risk of STIs appropriately sought PrEP care and were relatively early adopters of prophylactic treatment. It is also possible the observed effect may be an artifact of differential follow-up on versus off PrEP.”
Although uptake of PrEP may have led to a modest increase in diagnosis of STIs, especially of extragenital infections, the data suggest entry into PrEP care was associated with an overall reduced risk of STI, the authors stated, noting that this increase was likely multifactorial.
“Service members who sought PrEP may have recognized their existing high risk of HIV acquisition and adopted PrEP early and they may have benefited from greater STI prevention counseling. On the other hand, the observed reduction in overall risk of STI may have been an artifact of the longer surveillance period prior to initiation of PrEP where repeat asymptomatic undiagnosed infection may have reduced susceptibility to CT and NG infections,” the researchers wrote. “Nonetheless, the data suggest that routine STI screening should remain a cornerstone of PrEP care given the burden that increased STI rates have on public health, as well as the role that frequent follow-up and counseling can play in mitigating incident STIs. Long-term studies on STI incidence in the PrEP population could help inform policy and clinical guideline recommendations to mitigate risk.”
- Blaylock JM, Ewers EC, Bianchi EJ, King DB, et. Al. Risk of sexually transmitted infections among U.S. military service members in the setting of HIV pre-exposure prophylaxis use. PLoS One. 2023 Dec 28;18(12):e0296054. doi: 10.1371/journal.pone.0296054. PMID: 38153953; PMCID: PMC10754448.
