VA-Led Study Likely to Change Kidney Disease Treatment

Rajiv Agarwal, MD, a staff physician at the Roudebush VA and professor of medicine at Indiana University School of Medicine
INDIANAPOLIS — For years, clinicians have noted that inadequate evidence was available to promote the use of thiazide diuretics to treat hypertension in patients with advanced chronic kidney disease.
That likely changed with a new VA study published in The New England Journal of Medicine.
Richard L. Roudebush VAMC-led researchers determined that, among patients with advanced chronic kidney disease and poorly controlled hypertension, chlorthalidone therapy improved blood-pressure control at 12 weeks as compared with placebo.1
“Kidneys are key regulators of blood pressure. When an individual has chronic kidney disease, the kidneys are unable to control blood pressure,” said lead author Rajiv Agarwal, MD, a staff physician at the Roudebush VA and professor of medicine at Indiana University School of Medicine. “If a person suffers from chronic kidney disease and high blood pressure, it is more likely their kidney disease will advance even further and lead to other health issues, such as heart failure.”
Results from the CLICK study suggested that blood pressure was lowered by chlorthalidone by a meaningful 11 mm Hg at 12 weeks vs. 0.5 mm Hg reduction with placebo. In addition, the researchers pointed to a 50% reduction in albuminuria, which Agarwal said is especially significant, because that means chlorthalidone has the potential to reduce kidney failure progression and hospitalizations for heart failure.
Researchers randomly assigned patients with stage 4 chronic kidney disease and poorly controlled hypertension, as confirmed by 24-hour ambulatory blood-pressure monitoring, in a 1:1 ratio to receive chlorthalidone at an initial dose of 12.5 mg per day. Dosage was increased every four weeks, if necessary, to a maximum dose of 50 mg per day, or placebo. Randomization was stratified according to previous use of loop diuretics.
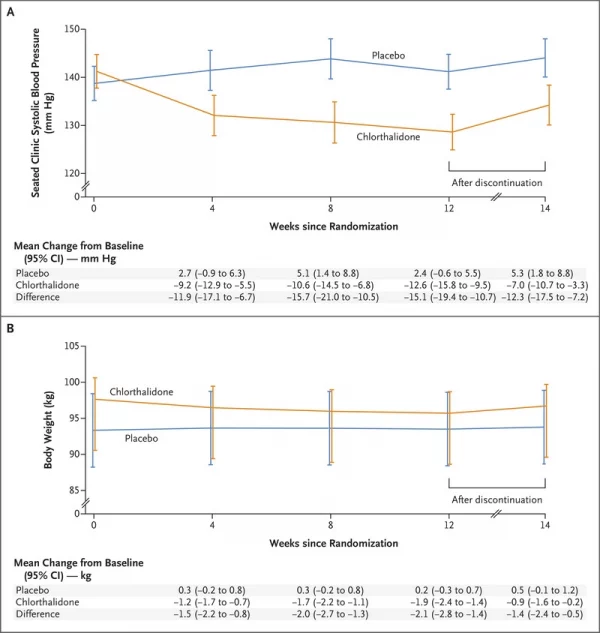
Click To Enlarge: Shown are the changes in the least-squares mean seated clinic systolic blood pressure (Panel A) and body weight (Panel B) over the 14-week trial period. At 12 weeks, the assigned regimen was discontinued. 𝙸 bars indicate 95% confidence intervals. The between-group difference was calculated as the value in the chlorthalidone group minus the value in the placebo group. Values are offset from each other at each time point for readability.
Defined as the primary outcome was the change in 24-hour ambulatory systolic blood pressure from baseline to 12 weeks, with secondary outcomes involving the change from baseline to 12 weeks in the urinary albumin-to-creatinine ratio, N-terminal pro-B-type natriuretic peptide level, plasma renin and aldosterone levels, and total body volume. Also assessed was safety.
The study team randomized 160 patients, of whom 121 (76%) had diabetes mellitus and 96 (60%) were receiving loop diuretics. The mean (±SD) estimated glomerular filtration rate was 23.2±4.2 ml per minute per 1.73 m2 of body-surface area at baseline, and the mean number of antihypertensive medications prescribed was 3.4±1.4.
At randomization, the authors report that the mean 24-hour ambulatory systolic blood pressure was 142.6±8.1 mm Hg in the chlorthalidone group and 140.1±8.1 mm Hg in the placebo group, with mean 24-hour ambulatory diastolic blood pressure of 74.6±10.1 mm Hg and 72.8±9.3 mm Hg, respectively.
In terms of 24-hour systolic blood pressure from baseline to 12 weeks, researchers calculated a between-group difference of -10.5 mm Hg (95% CI, -14.6 to -6.4) (P<0.001).
“The percent change in the urinary albumin-to-creatinine ratio from baseline to 12 weeks was lower in the chlorthalidone group than in the placebo group by 50 percentage points (95% CI, 37 to 60),” the researchers pointed out, adding that hypokalemia, reversible increases in serum creatinine level, hyperglycemia, dizziness and hyperuricemia occurred more frequently in the chlorthalidone group than in the placebo group.
Low-Cost Solution
“”These results show chlorthalidone is a low-cost solution for the treatment of hypertension in people with chronic kidney disease,” Agarwal explained in an Indiana University press release. “These are people who are already taking a variety of medicines, so to have one that is cheap and effective is incredibly meaningful. However, the drug is potent, so the lowest therapeutic dose and careful monitoring is needed to avoid complications.”
Background information in the article describes how hypertension, although a common risk factor for both cardiovascular disease and chronic kidney disease, is often poorly controlled, especially in patients with advanced chronic kidney disease.
“Thiazide or thiazide-like diuretics are important agents for lowering blood pressure in patients with essential hypertension,” the authors explained. “Chlorthalidone, a thiazide-like diuretic, reduces cardiovascular morbidity, such as the incidence of stroke and heart failure, and cardiovascular mortality. However, its efficacy and safety among patients with advanced chronic kidney disease remain poorly understood.”
On the basis of preliminary evidence of an effect on blood pressure in patients with chronic kidney disease, the study team hypothesized that, among patients with advanced chronic kidney disease and uncontrolled hypertension, chlorthalidone would decrease the 24-hour ambulatory systolic blood pressure. Also suggested was that chlorthalidone would reduce the degree of albuminuria over 12 weeks. The goal was to provide preliminary evidence that chlorthalidone is renoprotective and cardioprotective.
“The time course of the blood-pressure changes suggests that most of the reduction in blood pressure occurred within 4 weeks after therapy with 12.5 mg of chlorthalidone was initiated (in addition to other antihypertensive medications),” the authors wrote. “Reductions in body weight, body volume, and NT-proBNP levels and increases in plasma renin and aldosterone levels within 4 weeks after starting chlorthalidone therapy suggest that the mechanism of blood-pressure reduction is consistent with the changes in effective arterial blood volume over time, which decreased during the treatment period and then increased after the regimen was discontinued.”
They advised that, two weeks after chlorthalidone therapy was discontinued, 44% of the reduction in seated clinic systolic blood pressure and 53% of the peak weight loss that was observed at the end of the 12-week treatment period remained.
“The reported half-life of chlorthalidone is 45 to 60 hours, and the duration of action is even longer at 48 to 72 hours,” the researchers noted. “The persistent effects on blood pressure and weight loss are consistent with the long duration of action of chlorthalidone or a modifying effect of the drug on kidney disease. The large reduction in blood pressure is likely to have occurred because chlorthalidone is three times as potent as hydrochlorothiazide.”
The authors also posited, “The reversible changes in the estimated GFR that occurred in the chlorthalidone group were probably due to better blood-pressure control, which has been observed in other trials. At 2 weeks after chlorthalidone therapy was discontinued, the blood pressure remained below the baseline value, but the estimated GFR returned to approximately the baseline value, which suggests the additional involvement of tubuloglomerular feedback.”
The study recommended that chlorthalidone should be used with caution in patients receiving loop diuretics, primarily because of the risk of an increase in the serum creatinine level. “The lowest dose of chlorthalidone produced most of the blood-pressure-lowering effect, and this might be the safest dose to use. A reduction in the dose of the loop diuretic might be needed,” it recommends.
Chlorthalidone was approved by the Food and Drug Administration in 1960 for treatment of high blood pressure or hypertension. Over time, it came to be common wisdom that the drug was ineffective in treating high blood pressure in people with advanced chronic kidney disease.
- Agarwal R, Sinha AD, Cramer AE, Balmes-Fenwick M, Dickinson JH, Ouyang F, Tu W. Chlorthalidone for Hypertension in Advanced Chronic Kidney Disease. N Engl J Med. 2021 Nov 5. doi: 10.1056/NEJMoa2110730. Epub ahead of print. PMID: 34739197.