BUFFALO, NY — For veterans with multiple sclerosis (MS), the COVID-19 pandemic increased anxiety. Would the demyelinating disease increase the risk of hospitalization or death in patients infected with SARS-CoV-2? Would the immunomodulatory therapies frequently used to slow MS progression make patients even more vulnerable?
VA research provides reassurance for clinicians and their patients that veterans with MS receiving DMTs are not at greater risk; if anything, they appear to do better than patients without MS when hospitalized with COVID-19.
Early analyses demonstrated that immunocompromised patients faced a substantially greater risk of severe disease, with higher rates of hospitalization and death associated with COVID-19 infection. Studies found that patients taking immunosuppressants following organ or stem cell transplants or to treat cancer fared poorly, and individuals with weakened immune systems caused by human immunodeficiency virus (HIV) also had higher risk of severe disease and worse outcomes.
In light of these findings, clinicians initially recommended pausing disease-modifying therapies (DMTs) for patients with MS during the pandemic. At the same time, they worried about disease progression without the therapies.
A study published in August by researchers with the VA Western New York Healthcare System in Buffalo, N.Y., provided unexpected good news for MS patients. Remarkably, the team found that veterans hospitalized with COVID-19 who had multiple sclerosis and had active DMT prescriptions had better outcomes than patients without MS.1
“Results of this study suggest that not only is it safe to initiate or continue DMTs in MS patients during the COVID-19 pandemic, but it is also beneficial in the MS population given the decreased risk of COVID-19 mortality in addition to the established decreased risk of MS disease progression attributed to these therapies,” the researchers said.
The Study
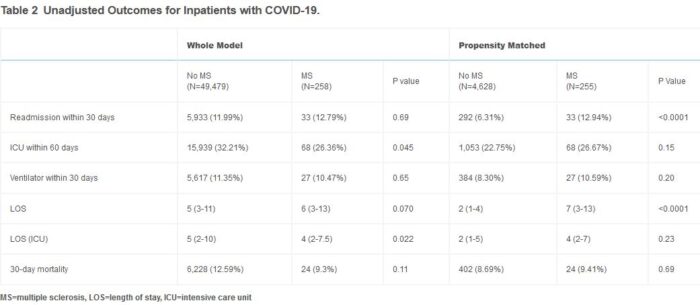
The retrospective study analyzed 49,737 cases of veterans hospitalized with COVID-19 at 125 VA facilities between March 3, 2020, and October 1, 2021, using the Corporate Data Warehouse. Of those, 255 had MS. The researchers evaluated all-cause mortality in these patients for 30 days after their initial positive COVID test.
For the comparator component of the study, the team propensity matched the 255 patients with MS to 4,628 veterans without MS on a 1:20 basis. Propensity matching adjusted for age, gender, race, morbidity, use of dexamethasone (as an indicator of COVID-19 severity) and ventilator use.
DMTs analyzed in the study included dimethyl fumarate, fingolimod, glatiramer, interferon 1A and 1B, natalizumab, siponimod, teriflunomide as well as other, less common, therapies. Ocrelizumab and rituximab were not included, as no veterans in the study were prescribed either therapy. Veterans were considered to be on a DMT if they had received a prescription for one within 90 days of a positive COVID-19 test or the record showed an inpatient medication order for a DMT.
Overall, coronary artery disease, heart failure, chronic kidney disease, cardiovascular disease, diabetes, and hypertension were less common in the MS cohort. Veterans with MS were also more likely to have been vaccinated. In the propensity score matched cohort, vaccination status did not differ between those with and without MS, but the difference in the listed chronic diseases persisted.
On an unadjusted basis, no statistically significant difference in 30-day mortality was found between veterans with MS (9.3%) and those without MS (12.6%), p=0.11. In the propensity score analysis, patients with MS had a 30-day mortality rate of 9.4% compared to 8.7% for those without MS, p=0.69.
Multivariable logistic regression analysis, however, revealed that disease-modifying therapies for MS “consistently reduced the odds of 30-day mortality,” the researchers wrote. In the whole model, DMTs reduced the odds of dying within 30 days by 84% (OR: 0.16 [95%CI: 0.01-0.82] p=0.023). In comparison, vaccination reduced the risk of death 59% (OR: 0.41 [95%CI: 0.37-0.44] p<0.0001). Similar results were seen in the propensity score matched cohort and in a sub-analysis of unvaccinated veterans.
“Following hospitalization for COVID-19 infection, MS patients who were taking DMTs (excluding anti-CD20 inhibitors) were over 5 times less likely to die from complications relating to COVID-19 infection (OR 0.18), accounting for other relevant factors, such as age, vaccination status, and comorbidities,” the VA team found.
While “MS patients were less likely to die than those in the general population when taking DMTs,” even without DMTs, MS patients had comparable mortality rates to other hospitalized patients without MS. “In the whole model, hospitalized patients with MS had less severe infection compared to the general population evidenced by the lower rates of ICU admission (26.4% vs 32.2%, p=0.045) and shorter mean length of time in the ICU (4 days vs 5 days, p=0.022),” the team added.
The researchers noted that, far from increasing the risk of severe disease and mortality, DMTs appear to provide protection in MS patients hospitalized with COVID-19. The team cautioned that the findings may not support the use of DMTs for COVID-19 treatment or prevention in patients who do not have MS, however, given the known risks of the therapies.
- Fuchs TA, Wattengel BA, Carter MT, El-Solh AA, Lesse AJ, Mergenhagen KA. Outcomes of Multiple Sclerosis Patients Admitted with COVID-19 in a Large Veteran Cohort. Mult Scler Relat Disord. Published online June 11, 2022. doi:10.1016/j.msard.2022.103964