
Jeffrey Cohen, MD, chief of the Laboratory of Infectious Diseases and chief of the Medical Virology Section at NIAID
BETHESDA, MD — Scientists at the National Institute of Allergy and Infectious Diseases (NIAID) and Walter Reed Army Institute of Research (WRAIR) have developed a panel of investigational monoclonal antibodies (mAbs) that target two key proteins—gH and gL—on the surface of the Epstein-Barr virus (EBV).
EBV is a common virus that is the cause of most cases of infectious mononucleosis and is associated with B-cell lymphomas, epithelial cell malignancies and other diseases.
The two proteins are known to facilitate EBV fusion with human cells and cause infection. In laboratory studies the investigational antibodies prevented infection of human epithelial and B cells. One of the six, called mAb 769B10, provided near-complete protection against viremia and lymphoma in a humanized mouse EBV challenge model, researchers reported in the Nov. 8, 2022, issue of Immunity.1
EBV affects more than 90 percent of the human population. The most common result of infection is a case of infectious mononucleosis, which for most people resolves in two to four weeks. But 10 to 20 percent will have fatigue for several months, said Jeffrey Cohen, MD, chief of the Laboratory of Infectious Diseases and chief of the Medical Virology Section at NIAID. As such, it is a major case of lost time for military recruits, he said.
Less commonly, but importantly, EBV infection is associated with several cancers including Hodgkin lymphoma, gastric carcinoma, nasopharyngeal carcinoma and Burkitt lymphoma. “About 200,000 cases of cancer worldwide each year are associated with EBV with 140,000 deaths due to these cancers,” Cohen said told U.S. Medicine. EBV also appears to be a co-factor in development of multiple sclerosis (MS).
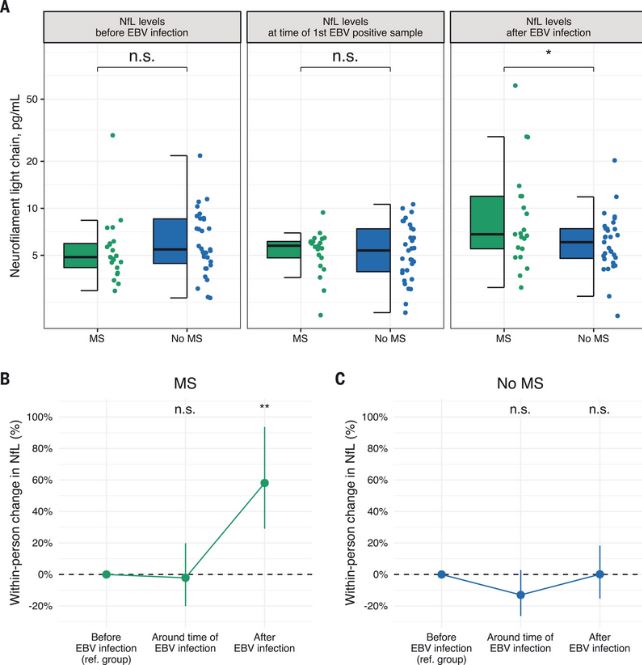
Click to Enlarge: (A) Box plots of sNfL levels before, around, and after the time of EBV infection. *P < 0.05, two-sided multivariable linear regression model adjusted for age and sex. (B) Within-person increase in sNfL levels in MS cases around and after time of EBV infection compared with before EBV infection. **P < 0.01, two-sided linear mixed-effects regression model. (C) Within-person increase in sNfL levels in controls around and after time of EBV infection compared with before EBV infection. Error bars in (B) and (C) are 95% CIs. sNfL levels increased significantly more in MS cases than in controls in the sample collected after time of EBV infection compared with before EBV infection (P < 0.001, two-sided linear mixed-effects regression model). Source: Science
In a study last year in Science, researchers found an association between EBV and MS in a cohort compromising more than 10 million young adults on active duty in the US military, 955 of whom were diagnosed with MS during their period of service. Risk of MS increased 32-fold after infection with EBV, they found, but was not increased after infection with other viruses, including the similarly transmitted cytomegalovirus. Serum levels of neurofilament light chain, a biomarker of neuroaxonal degeneration—one of the hallmarks of clinical deterioration in progressive multiple sclerosis—increased only after EBV seroconversion. “These findings cannot be explained by any known risk factor for MS and suggest EBV as the leading cause of MS,” the authors wrote. 2
It has been hypothesized that vaccination against EBV could prevent the development of diseases associated with it. Cohen said the new research may be an important step toward a vaccine—specifically against lymphoma in immunocompromised patients.
He said mAb 769B10 has the potential to be used to prevent infection by the virus in patients who have not been infected and who are at risk of developing EBV lymphoma after transplantation or in those with severe immune impairment—similar to the “bubble boy” who died after lymphoma of getting infected with EBV.
“By determining how the potent antibody we identified binds to the glycoprotein on the virus, it may help us to identify a vulnerable site of the glycoprotein that could be targeted by a vaccine,” he said.
Cohen, who has devoted 30 years to the study of EBV, said there are two EBV vaccines in clinical testing now. “We are testing one of these vaccines at the NIH Clinical Center in Bethesda at present,” he said, adding that that vaccine is derived directly from research he did with M. Gordon Joyce, PhD, when Joyce worked for the NIH Vaccine Research Center. Joyce is now chief of the Structural Biology group within the WRAIR’s Emerging Infectious Diseases Branch, but the collaboration of the scientists on EBV continues.
The other vaccine was developed by Moderna, he said. Both of the other vaccines were produced before the antibody work the group published in their current paper, he said.
The group plans to do additional research on mAb 769B10 and its implications.
- Chen WH, Kim J, Bu W, Board NL, et. al. Epstein-Barr virus gH/gL has multiple sites of vulnerability for virus neutralization and fusion inhibition. Immunity. 2022 Nov 8;55(11):2135-2148.e6. doi: 10.1016/j.immuni.2022.10.003. Epub 2022 Oct 27. PMID: 36306784.
- Bjornevik K, Cortese M, Healy BC, Kuhle J, et. al. A. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022 Jan 21;375(6578):296-301. doi: 10.1126/science.abj8222. Epub 2022 Jan 13. PMID: 35025605.

