DURHAM, NC—Recent Food and Drug Administration approvals of chimeric antigen receptor (CAR) T cell therapies and novel immunotherapies have helped to significantly improve outcomes for patients with relapsed and refractory diffuse large B cell lymphoma (R/R DLBCL). But those advantages are not across-the board.
“Unfortunately, studies show persistent disparities in DLBCL that impact survival, including patient race, income and rural residence,” explained researchers from the Duke University School of Medicine, the Durham VAMC, both in Durham, and the VA’s National Oncology Program in Washington, DC.
Their presentation at the recent American Society of Hematology’s 65 Annual Meeting and Exposition in San Diego pointed out that the VA is a single-payer healthcare system that provides coverage for cancer therapies and travel support, which show promise in addressing barriers to treatment.1
Source: Scobie MR, Anderson CE, Friedman DR, Kelley MJ, Rodgers TD. (2023, Dec. 9-12) Impact of Social Determinants of Health and Novel Therapies on the Outcomes of Relapsed and Refractory Diffuse Large B Cell Lymphoma. 65th American Society of Hematology Annual Meeting and Exposition, San Diego, California.
The study team, using national VA data, examined the influence of social determinants of health (SDoH) and treatment patterns on the outcomes of veterans with R/R DLBCL in the era of novel therapies.
To do that, the researchers identified patients receiving frontline anthracycline at any time and any systemic therapy for R/R DLBCL from 2019-2022; the VA Corporate Data Warehouse and retrospective chart review were used to gather the data.
In addition, the Social Deprivation Index (SDI) was estimated at the ZIP code level using the 2019 American Community Survey. Median household income was estimated from federal census tract data.
Meeting study eligibility were 225 patients across 70 VAMCs; their median age was 69, and most (96%) were male, reflecting the VA population. The median follow-up was 32.7 months (range, 2.3-217.5).
Participants received a median of three lines of therapy (range, 2-8). Novel immunotherapies were administered to 98 patients (44%): 10 (4%) received loncastuximab, 23 (10%) tafasitamab, and 83 (37%) polatuzumab. 52 (23%) and 26 (12%) patients received CAR T and autologous stem cell transplant (auto-SCT), respectively.
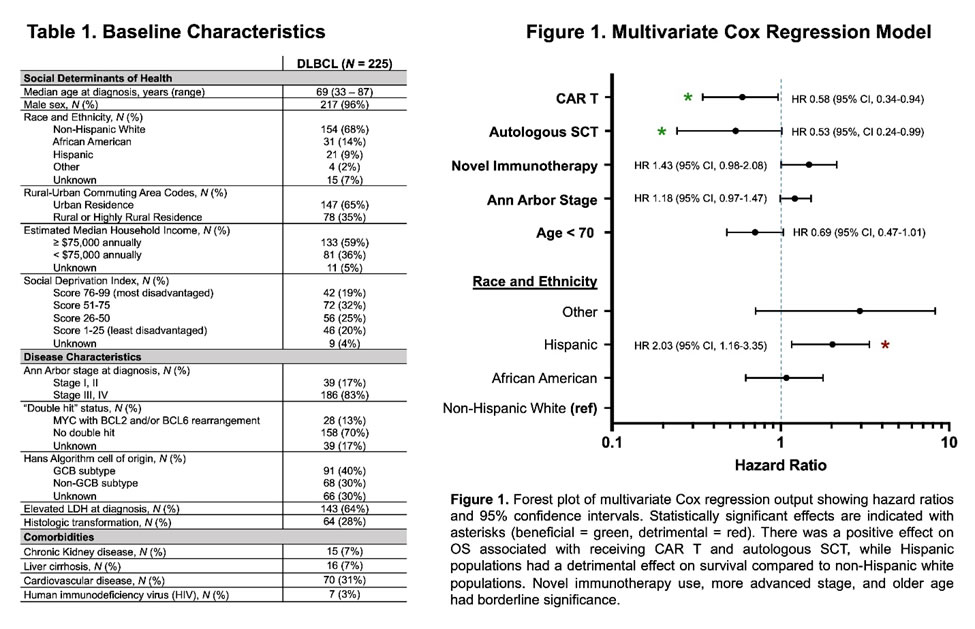
Results indicated that the estimated 2-year overall survival (OS) was 65.8% (95% CI, 59.1-71.7). In univariate analyses, age less than 70 (HR 0.58, 95% CI 0.41-0.82), use of CAR T (HR 0.64, 95% CI 0.40-0.99), and auto-SCT (HR 0.39, 95% CI 0.19-0.73) were associated with improved overall survival (OS).
“Interestingly, the use of novel immunotherapies was associated with worse OS (HR 1.43, 95% CI 1.01-2.01), but among those who received it, using it in earlier lines had better survival (HR 0.71, 95% CI 0.54-0.94,” the authors advised. “Notably, Hispanic white had worse survival than non-Hispanic white patients (HR 1.97, 95% CI 1.13-3.23), while African Americans had similar survival to non-Hispanic white patients. Higher stage showed worse survival, but this did not reach significance (HR 1.12, 95% CI 0.93-1.40).”
Other SDoH (sex, income, rural residence, SDI), disease traits (transformed, LDH, cell of origin, double-hit), and medical comorbidities were not found to affect survival.
In multivariate analysis, the impacts of CAR T, auto-SCT and Hispanic ethnicity persisted. Among the 143 patients receiving at least three lines of therapy, the only significant covariate in multivariate regression for OS was CAR T (HR 0.57, 95% CI 0.32-0.96), the researchers pointed out.
They recounted how referrals for SCT and CAR T were sent for 65 (29%) and 81 (36%) patients, respectively. “The most advantaged SDI quartile had higher odds of getting an SCT referral than the least advantaged quartile (OR 5.00, p = 0.0015),” according to the study. “Similarly, household income ≥ $75,000 had higher odds of SCT referral vs. lower incomes (OR 2.88, p = 0.004). Race did not affect SCT referral rates, and SDoH in general did not impact CAR T referrals. SDoH was not associated with whether patients actually received novel immunotherapies, CAR T, or SCT (only affecting referral rates in the latter).”
In fact, the authors pointed out, the most common reasons for not pursuing auto-SCT after referral were:
- comorbidities (n= 23, 33%),
- pursuance of other cell therapies (n= 17, 25%), and
- patient decision (n= 12, 17%).
The most common reasons for deferring CAR T were:
- comorbidities (n= 15, 28%),
- death (n= 15, 28%), and
- patient decision (n= 6, 11%).
At last follow-up, of the 47 patients in remission for more than a year, 27 (57%) had CAR T or SCT as their last therapy.
“Cellular approaches were the most impactful factors in improving OS. Hispanic ethnicity remained an independent poor prognostic factor, but other SDoH did not impact survival in this cohort,” the researchers concluded. “Broad drug coverage by a single-payer system, front-line anthracycline, and equal receipt of cell therapies may have mitigated some of the impact of SDoH on DLBCL survival. Nonetheless, cell therapies had a high referral but low administration rate, highlighting the challenge of getting patients to definitive treatment.”
- Scobie MR, Anderson CE, Friedman DR, Kelley MJ, Rodgers TD. (2023, Dec. 9-12) Impact of Social Determinants of Health and Novel Therapies on the Outcomes of Relapsed and Refractory Diffuse Large B Cell Lymphoma. 65th American Society of Hematology Annual Meeting and Exposition, San Diego, California.

