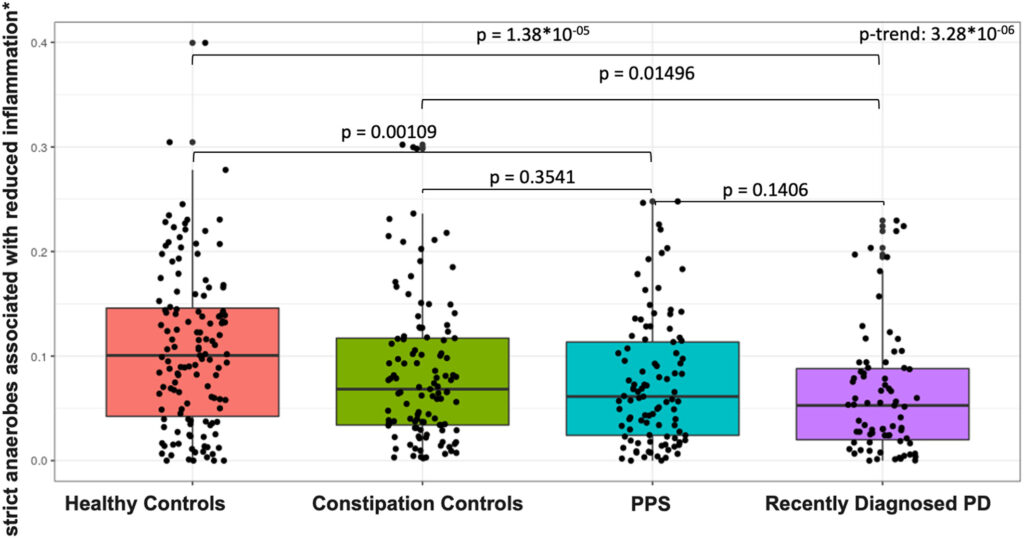
Click to Enlarge: Abundance of strict anaerobes associated with reduced inflammation by study group. Total (sum) abundance, stratified by phenotype group, of strict anaerobes associated with production of short-chain fatty acids. Sums include abundance of Eubacterium rectale, Roseburia inulinivorans, Roseburia intestinalis, Eubacterium hallii, Anaerostipes hadrus, and Faecalibacterium prausnitzii.61 P values (nominal) are reported for pair-wise Wilcoxon tests. We tested for linear trend across phenotype groups by fitting a logistic regression model to ordinal phenotype (healthy controls, constipation controls, patients with PPS, and patients with PD) in relation to summed species abundance adjusting for sequencing batch, age, pack-years of smoking, BMI, and MED diet adherence. BMI = body mass index; MED = Mediterranean Dietary Pattern; PD = Parkinson’s disease; PPS = prodromal Parkinson’s syndrome Source: Annals of Neurology
BEDFORD, MA — Parkinson’s disease (PD) is a complex neurodegenerative disorder that affects approximately 1% of the population age 65 and older and an estimated 110, 000 U.S. veterans. Research has suggested that the gut microbiome, a diverse community of microorganisms residing in the intestines, might play a role in PD’s development; however, studies exploring the connection have generated conflicting results.
A new study led by a VA researcher provided important clues to the role of the gut microbiome in PD, focusing on prodromal (premotor) PD and utilizing advanced metagenomic profiling. The research, conducted as a nested case-control study within two large epidemiological cohorts, offers intriguing insights into potential early biomarkers for PD.
In the new study, published in Annals of Neurology, researchers led by Natalia Palacios, ScD, of Edith Nourse Rogers Memorial VA Hospital in Bedford, MA, profiled the fecal metagenomes of 420 participants in the Nurses’ Health Study and the Health Professionals Follow-up Study. Participants included those with recent-onset PD, individuals displaying features of prodromal PD, controls with constipation and healthy controls. The aim was to identify both microbial taxonomic and functional features associated with PD and those suggestive of prodromal PD. Comprehensive analyses were carried out, assessing bacterial species and pathways associated with both prodromal and recently onset PD.1
The study’s primary findings indicate that the gut microbiome of individuals with prodromal PD exists on a continuum between the microbiomes of healthy individuals and those with diagnosed PD. By scrutinizing microbial profiles, the research team identified a range of bacterial species and microbial pathways that were notably different in both prodromal PD and recently diagnosed PD patients compared to healthy controls. This suggests that the microbial changes in PD may precede the clinical diagnosis, potentially paving the way for prodromal PD biomarkers that encompass microbiome features.
Some, although not all, microbial alteration observed in the study are consistent with a state of elevated intestinal inflammation in PD, the study found.
Linked With IBD
“Gastrointestinal disturbance has been recognized as a prodromal feature of PD and is present in up to 80% of patients,” Palacios and her colleagues wrote. Inflammatory gastrointestinal conditions, such as inflammatory bowel diseases (IBDs), have been associated with an increased risk of PD in several large cohort studies. IBD and PD have been shown to share genetic risk factors. Further, intestinal inflammation has been shown to lead to onset or worsening of PD-related changes in animal models. In human studies, levels of pro-inflammatory cytokines, including TNF-α, interferon-y, IL-1, IL-1β, and CRP have been shown to be elevated in PD compared to controls, suggestive of intestinal immune dysregulation and inflammation in PD.
“Unlike prior studies that have also reported an inflammatory microbiome state in patients with PD compared to controls, our work goes further, demonstrating that [microbiome imbalance] reflective of immune activation is present at the earliest stages of PD, and may thus play an important role in PD pathogenesis,” they wrote. “This provides additional evidence in favor of a causal role of gastrointestinal inflammatory changes in PD pathogenesis.”
One of the most-promising aspects of this research is the development of a microbiome-based classifier capable of discriminating between individuals with recently diagnosed PD and healthy controls. This classifier shows potential as a biomarker for PD, even when applied to individuals showing signs of prodromal PD. Importantly, this suggests that the microbial changes observed are not merely a result of post-diagnosis factors, such as medication or lifestyle changes, but may be an integral part of the pathological process underlying PD.
A better understanding of that pathological process and its early identification has potential for prevention and treatment. “The microbiome is modifiable, and several studies have demonstrated proof-of-concept that microbiota manipulation could contribute to PD prevention and treatment. Identification of PD-specific microbial features could lead to future therapies to treat PD or slow its progression,” the authors wrote. “In addition to their potential for preventing or slowing the progression of PD, microbiome-derived therapies could also serve to improve response to existing PD treatment. Bacteria play an important role in metabolism of levodopa, the key medication used to treat PD symptoms, suggesting that altering this metabolism may impact drug efficacy.”
The authors said a key strength and unique contribution of their study is its employment of rigorously designed prospective cohorts. “Drawing cases and controls from the same population substantially reduces the potential for selection bias in our comparisons. This is in contrast to most previous studies, which relied on a traditional case-control study design that is prone to this type of bias. Furthermore, to our knowledge, ours is the first study that focuses on the microbiome in a well-defined group of patients with prodromal PD.”
Still, they acknowledge larger, longitudinal studies that follow healthy participants through the prodromal phase to PD onset are needed and more definitively confirm the findings of their study.
- Palacios N, Wilkinson J, Bjornevik K, Schwarzschild MA, McIver L, et al. Metagenomics of the Gut Microbiome in Parkinson’s Disease: Prodromal Changes. Ann Neurol. 2023 Sep;94(3):486-501. doi: 10.1002/ana.26719. Epub 2023 Jul 8. PMID: 37314861; PMCID: PMC10538421.
