Enables Memory Recall Without ‘PTSD-related Shame, Anger’
SAN FRANCISCO — Treatment with methylenedioxymethamphetamine (MDMA) showed far greater effectiveness than usual care in a group of patients with post-traumatic stress disorder, according to a new study.
Study participants, including veterans who developed PTSD after combat experience, improved even if their PTSD was severe or they had associated comorbidities such as dissociative PTSD, depression, history of alcohol and substance use disorders and childhood trauma, concluded the study in Nature Medicine. Most, 82%, had comorbid major depression.1
“Not only is MDMA-assisted therapy efficacious in individuals with severe PTSD, but it may also provide improved patient safety,” explained the University of California San Francisco-led authors. “Compared with current first-line pharmacological and behavioral therapies, MDMA-assisted therapy has the potential to dramatically transform treatment for PTSD and should be expeditiously evaluated for clinical use.”
Researchers pointed out that PTSD is a major public health problem, but currently available treatments are only modestly effective. Their randomized, double-blind, placebo-controlled, multisite Phase 3 clinical trial was designed to test the efficacy and safety of MDMA-assisted therapy.
To do that, they randomized 90 participants who had washed out on Food and Drug Administration-approved psychiatric medications, 1:1 to receive manualized therapy with MDMA or with placebo, combined with three preparatory and nine integrative therapy sessions.
Of those, 17.8% were identified as veterans, and 12.2% said they had experienced military combat. The overall group was smaller than originally planned because of limitations associated with COVID-19 pandemic.
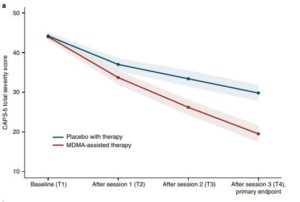
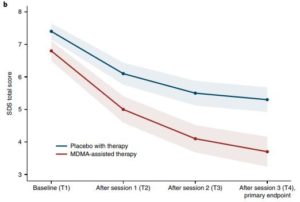
A range of tests—the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5, the primary endpoint) and functional impairment, measured with the Sheehan Disability Scale (SDS, the secondary endpoint) —were used to assess the patients at baseline and at two months after the last experimental session.
Results indicated that MDMA induced “significant and robust attenuation in CAPS-5 score compared with placebo (P < 0.0001, d = 0.91) and to significantly decrease the SDS total score (P = 0.0116, d = 0.43),” according to the researchers.
Mean change in CAPS-5 scores in participants completing treatment was -24.4 (s.d. 11.6) in the MDMA group and -13.9 (s.d. 11.5) in the placebo group.
Yet, the authors emphasized, MDMA did not induce adverse events of abuse potential, suicidality or QT prolongation. Some of those problems have been reported in illegal users of the drug compound, which has street names of Ecstasy and Molly.
“PTSD is a common and debilitating condition with immeasurable social and economic costs that affects the lives of hundreds of millions of people annually,” the authors wrote. “There are a number of environmental and biological risk factors that contribute to the development and maintenance of PTSD, and poor PTSD treatment outcomes are associated with several comorbid conditions that include childhood trauma, alcohol and substance use disorders, depression, suicidal ideation and dissociation. It is therefore imperative to identify a therapeutic that is beneficial in those individuals with the comorbidities that typically confer treatment resistance.”
The study noted that selective serotonin reuptake inhibitors (SSRIs) sertraline and paroxetine are FDA-approved first-line therapeutics for the treatment of PTSD, but that as many as 60% of patients with the condition do not respond to those medications. It also described how, although evidenced-based trauma-focused psychotherapies such as prolonged exposure and cognitive behavioral therapy are considered to be the gold standard treatments for PTSD, failure to respond or dropping out are both common among PTSD patients whose symptoms continue.
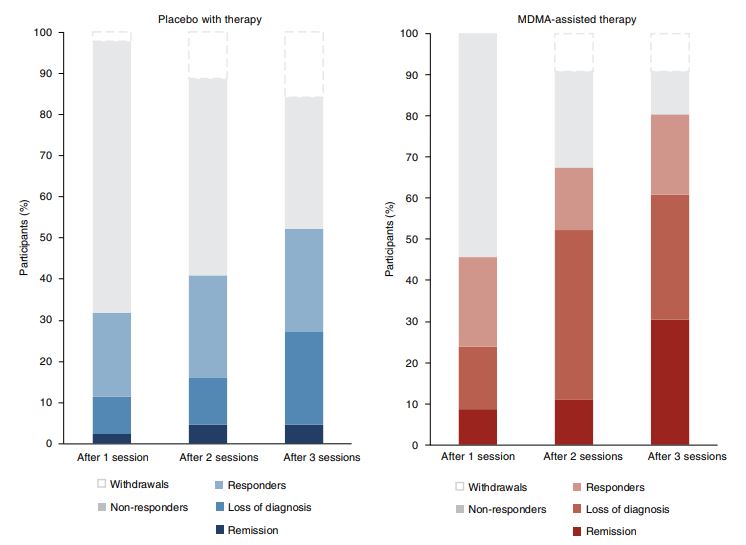
Click to Enlarge: Treatment response and remission for MDMA and placebo groups as a percentage of total participants randomized to each arm (MDMA,
n= 46; placebo, n= 44). Responders (clinically significant improvement, defined as a ≥10-point decrease on CAPS-5), loss of diagnosis (specific
diagnostic measure on CAPS-5), and remission (loss of diagnosis and a total CAPS-5 score of ≤11) were tracked in both groups. Non-response is defined
as a <10-point decrease on CAPS-5. Withdrawal is defined as a post-randomization early termination.
“Novel cost-effective therapeutics are therefore desperately needed,” according to the researchers, who said they opted for MDMA, which causes serotonin release by binding primarily to presynaptic serotonin transporters. Background information in the study explained that MDMA has been shown to aid fear memory extinction, modulate fear memory reconsolidation – possible because of an oxytocin-dependent mechanism — and improve social behavior in animal models.
The authors advised that pooled analysis of six Phase 2 trials of MDMA-assisted therapy for PTSD have now demonstrated promising safety and efficacy findings.
MDMA-assisted therapy for PTSD was granted an FDA Breakthrough Therapy designation, and the study’s protocol and statistical analysis plan (SAP) were developed in conjunction with the FDA.
The nonprofit Multidisciplinary Association for Psychedelic Studies (MAPS), which sponsored the current study and provided the MDMA for research, sought that designation from the FDA, and it was granted in 2017.
MAPS and the FDA also reached agreement under the Special Protocol Assessment Process (SPA) for the design of the trials of MDMA-assisted psychotherapy for patients with severe PTSD. By granting Breakthrough Therapy Designation, the FDA has agreed that this treatment may have a meaningful advantage and greater compliance over available medications for PTSD.
“Reaching agreement with FDA on the design of our Phase 3 program and having the ability to work closely with the agency has been a major priority for our team,” said Amy Emerson, executive director of the MAPS Public Benefit Corp. (MPBC). “Our Phase 2 data was extremely promising with a large effect size, and we are ready to move forward quickly. With breakthrough designation, we can now move even more efficiently through the development process in collaboration with the FDA to complete Phase 3.”
MAPs announced at the time that, in its completed Phase 2 trials with 107 participants, 56% no longer qualified for PTSD diagnosis after treatment with MDMA-assisted psychotherapy, measured two months later. At the 12-month follow-up, 68% no longer had PTSD, according to the group, although most participants received just two or three sessions of MDMA-assisted psychotherapy. All had chronic, treatment-resistant PTSD and had suffered from PTSD for an average of 17.8 years.
Mitigated Depressive Symptoms
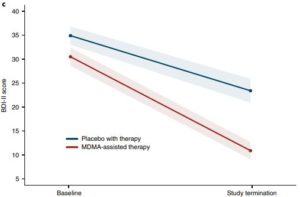
In the current study, researchers not only found MDMA treatment to be effective for PTSD, they also reported that it mitigated depressive symptoms as assessed using the Beck Depression Inventory-II. Furthermore, they wrote, MDMA did not increase the occurrence of suicidality during the study.
“These data illustrate the potential benefit of MDMA-assisted therapy for PTSD over the FDA-approved first-line pharmacotherapies sertraline and paroxetine, which have both exhibited smaller effect sizes in pivotal studies,” the authors emphasized. “Previous comparison of change in CAPS score between sertraline and placebo showed effect sizes of 0.31 and 0.37. Similarly, comparison of change in CAPS score between paroxetine and placebo showed effect sizes of 0.56, 0.45 and 0.09. By contrast, the effect size of 0.91 demonstrated in this study between MDMA-assisted therapy and placebo with therapy was larger than that for any other previously identified PTSD pharmacotherapy.”
More studies are needed, however, according to researchers, who called for a head-to-head comparison of MDMA-assisted therapy with SSRIs for PTSD.
“Although the present study tested the effects of MDMA using a model in which both treatment groups received supportive therapy, participants who received MDMA and supportive therapy (d = 2.1) had greater improvement in PTSD change scores compared with those who received placebo with supportive therapy (d = 1.2), suggesting that MDMA enhanced the effects of supportive therapy. In clinical practice, both MDMA and supportive therapy will be components of this PTSD treatment,” they added.
A potential downside was that previous research on MDMA for PTSD had suggested that those with a recent history of SSRI treatment might not respond as strongly to MDMA. The authors pointed out, “Given that 65.5% of participants in the current trial have a lifetime history of SSRI use, it is difficult to separate the ramifications of long-term SSRI treatment from the effects of treatment resistance. However, there was no obvious effect of previous SSRI use on therapeutic efficacy in this trial. Similarly, although years of PTSD diagnosis or age of onset may affect treatment efficacy, no obvious relationship was seen here between duration or onset of PTSD diagnosis and treatment efficacy.”
They posited that the therapeutic effects of MDMA might be related to a well-conserved mechanism of amygdalar serotonergic function that regulates fear-based behaviors and contributes to the maintenance of PTSD.
“Perhaps by reopening an oxytocin-dependent critical period of neuroplasticity that typically closes after adolescence, MDMA may facilitate the processing and release of particularly intractable, potentially developmental, fear-related memories,” researchers suggested, adding, “It is intriguing to speculate that the pharmacological properties of MDMA, when combined with therapy, may produce a ‘window of tolerance,’ in which participants are able to revisit and process traumatic content without becoming overwhelmed or encumbered by hyperarousal and dissociative symptoms. MDMA-assisted therapy may facilitate recall of negative or threatening memories with greater self-compassion and less PTSD-related shame and anger.”
The drug also might improve therapeutic alliance, which could help both treatment and outcomes, according to the study group, which noted, “Indeed, clinicians have suggested that “MDMA may catalyze therapeutic processing by allowing patients to stay emotionally engaged while revisiting traumatic experiences without becoming overwhelmed.”
Of special note to VA clinicians, the study pointed out that, while PTSD is a strong predictor of disability in both veteran and community populations, reduction in PTSD and depression symptoms led to significant improvement in patients’ ability to work, go to school and function well socially and within their families.
“Approximately 4.7 million U.S. veterans report a service-related disability, costing the U.S. government approximately $73 billion per year,” the authors stated. “Identification of a PTSD treatment that could improve social and family functioning and ameliorate impairment across a broad range of environmental contexts could provide major medical cost savings, in addition to improving the quality of life for veterans and others affected by this disorder.”
No major safety issues were reported in the MDMA arm of the study, according to the report, which added, “These data suggest that MDMA has an equivalent, if not better, safety profile compared with that of first-line SSRIs for the treatment of PTSD, which are known to carry a low risk of QT interval prolongation.”
The authors also pointed out that the timing of their findings is especially fortuitous, writing, “We may soon be confronted with the potentially enormous economic and social repercussions of PTSD, exacerbated by the COVID-19 pandemic. Overwhelmingly high rates of psychological and mental health impairment could be with us for years to come and are likely to impart a considerable emotional and economic burden. Novel PTSD therapeutics are desperately needed, especially for those for whom comorbidities confer treatment resistance.”
- Mitchell, J.M., Bogenschutz, M., Lilienstein, A. et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med (2021). https://doi.org/10.1038/s41591-021-01336-3