The debate continues a half-century after Agent Orange use ended over whether exposure to the herbicide in Vietnam and elsewhere contributed to the development of acute myeloid leukemia and myelodysplastic syndromes. A recent article from Yale researchers raised the issue anew.
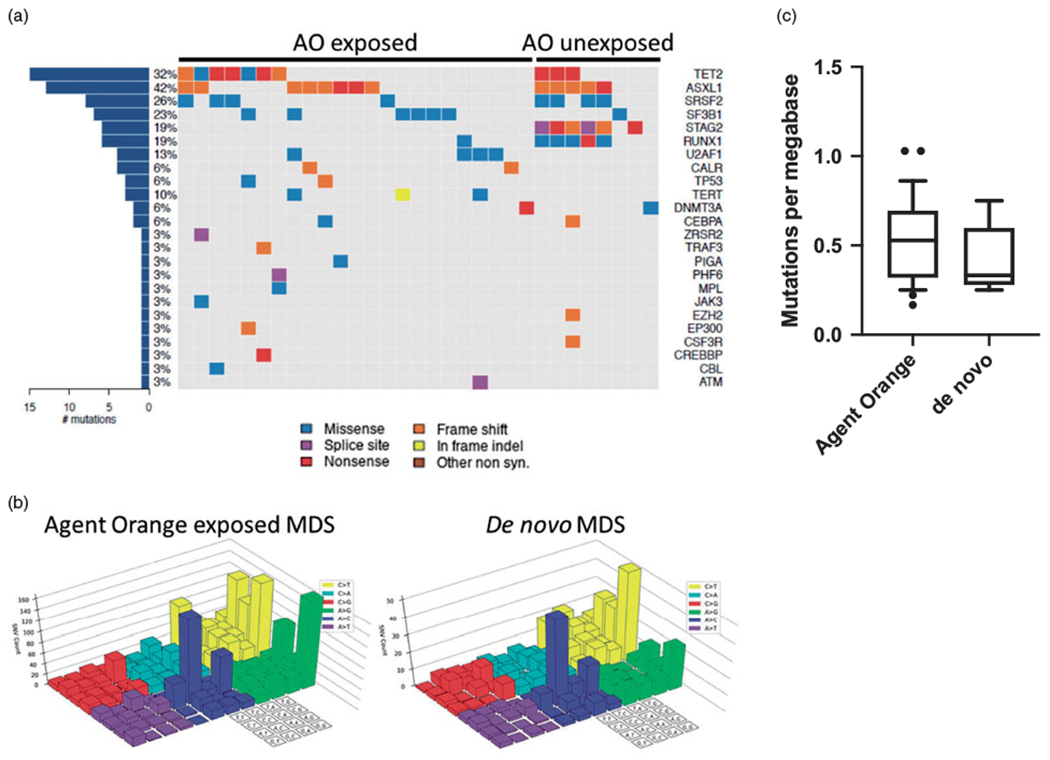
Click To Enlarge: The mutational spectrum is similar between Agent Orange exposed and control MDS patients. (a) Co-mutation plot for driver mutations identified in Agent Orange exposed and control subjects. Syn: synonymous. (b) Mutational signature analysis for subjects exposed to Agent Orange and controls. Colored chart signifies the specific single nucleotide variant and 4 × 4 grid denotes the sequence context. (c) Mutational burden per Mbp for Agent Orange exposed veterans compared to controls. Source: Leuk Lymphoma. 2020 Mar;61(3):728-731.
NEW HAVEN, CT — The Vietnam War ended 47 years ago, and the use of the herbicide Agent Orange was banned by the United States 51 years ago, but debate continues over whether acute myeloid leukemia and its often-related condition, myelodysplastic syndromes, should become presumptive conditions related to Agent Orange exposure.
As has been the case for decades, research results have been mixed on the question of whether AML and MDS in veterans is caused by toxic exposures during military service.
The issue was raised anew when, earlier this year, two prominent specialists from Yale University School of Medicine and Yale Cancer Center wrote in the journal Leukemia & Lymphoma, “Agent Orange (AO) was the dominant weaponized herbicide employed by the United States (US) military during the Vietnam war. AO, however, was found to be regularly contaminated by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), the most toxic dioxin known; furthermore, AO was commonly diluted in the field with other aromatic hydrocarbons to assist with delivery mechanisms.”
The authors, Rory M. Shallis, MD, and Steven D. Gore, MD, went on to state, “Unbeknownst to the U.S. military and the millions exposed, these events have likely contributed to the development of acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) that has affected many veterans.”
Shallis and Gore argued that studies failing to find an association between AO exposure and AML/MDS are limited in their methodology and application. “The acknowledgement that the known carcinogen TCDD was a contaminant in AO when paired with a strong biological plausibility for its leukemogenicity and an observed increased risk of AML/MDS in TCDD-exposed individuals should suffice to establish causal association and that veterans to whom this might apply should be awarded appropriate indemnity,” they concluded.
The VA’s presumptive list already includes chronic B-cell leukemias, added in 2003, as well as the blood cancer multiple myeloma, added in 2016. So far, however, AML and MDA have not been added to the list. (AML is technically not a -cell lymphoma, although past studies have suggested that AML patients have elevated rates of a type of B cells, Breg cells and that might reveal poor prognosis.
“It is notable that numerous hematologic malignancies—chronic B cell leukemias, multiple myeloma, light-chain amyloidosis, Hodgkin disease, and non-Hodgkin lymphoma—and some other cancers are on the “presumptive” list, but not MDS or acute myeloid leukemia,” David Steensma, MD, of the Dana-Farber Cancer Institute in Boston wrote on the Aplastic Anemia & MDS International Foundation website.
The American Cancer Society reports that about 20,050 new cases of AML occur each year, mostly in adults. Nearly all of the 11,540 deaths are in adults, it adds.
While AML is one of the most common types of leukemia in adults—and the most common type of acute leukemia—it still accounts for only about 1% of all cancer cases. The deadly cancer also is a disease of older people and is uncommon before the age of 45, with an average age of diagnosis of about 68. It does occur in children sometimes, however.
One study raised the possibility that AML in veterans might be linked to exposure of military toxicities in general, not just Agent Orange and that those exposures affected survival rates.
For the presentation at the 2016 American Society of Clinical Oncology Annual Meeting, researchers from the Oklahoma University Health Sciences Center retrospectively analyzed all adult patients diagnosed with AML and treated at the University of Oklahoma from January 2000 to June 2012. Responses were received from 181 patients or next-of-kin, with most, 61%, men with a median age of 52. 2
Those patients had a median overall survival of 27 months, and most patients reported good family support (65%), having not served in the military (94%), or having difficulty with travel (73%).
After adjusting for age and cytogenetic risk, the researchers found that military status was marginally associated with OS (p-value = 0.0548). “The hazard of death for those in the military is 2.36 times higher for patients with AML relative to civilians (95% CI: 0.98, 5.69),” the authors noted.
Researchers concluded that patients “who served in the military may have lower OS compared to those who have not. This finding may be related to unmeasured exposure to potential carcinogens,” and called for more research on the issue.
Myelodysplastic Syndromes
A 2020 study from the Dana-Farber Cancer Institute and the Broad Institute of MIT and Harvard, both in the Boston area, looked at myelodysplastic syndromes (MDS) occurring in Agent Orange exposed individuals carrying a mutational spectrum similar to that of de novo MDS. It did not find clear evidence that toxic exposure led to the condition.3
Characterized by blood cytopenias, dysplastic hematopoietic differentiation and a risk of progression to AML, MDS include a group of clonal hematopoietic disorders. Most cases have no indisputable cause or exposure and are considered, “de novo MDS.” On the other hand, the authors of a 2020 study published in Blood noted that several causative agents have been linked to the development of MDS, called “therapy-related MDS”, including organic solvents such as benzene, ionizing radiation and cytotoxic chemotherapy.
In a 2020 article in Blood, Washington University School of Medicine researchers estimated that about 30% of MDS patients eventually progress to AML, “which is diagnosed by an increase in blast count to ≥20% of total nucleated cells in the bone marrow and is commonly termed “secondary AML to MDS. Secondary AML accounts for up to 25% to 35% of total AML cases, with most (60-80%) arising from an antecedent MDS.”4
The Dana-Farber-led researchers explained that Agent Orange (AO) was a phenoxy herbicide mixture used extensively by the United States military as a strategic defoliant. During the Vietnam War, when it was used the most, it was commonly diluted in the field by military staff with kerosene, gasoline, or JP-4 jet fuel to facilitate aerosolization and spraying from aircraft.
“While no clear link between the development of MDS and exposure to AO has yet been documented, the long latency for development and relative rarity of MDS has made this difficult to assess in epidemiological studies, and the potential hematopoietic toxicity of AO has raised the possibility of an association,” the authors wrote.
To determine if MDS that developed in AO-exposed individuals has a common etiologic link, researchers performed next-generation exome sequencing to assess acquired somatic mutations in a panel of 29 Vietnam-era military veterans diagnosed with MDS and who had prior reported exposure to AO that met the VA definition of presumptive AO exposure.
Eligible veterans, who had a median age of 65 and were all white males, were asked to complete an online form and to provide a peripheral blood sample. Most of the 29 patients included in the study had served in the Army. In comparison, the study team examined the exome sequences of nine patients with MDS without known military service or suspected AO exposure and also used a dataset of more than 3,000 patients with MDS assessed with a 95-gene molecular profiling tool.
“The driver mutations identified in the AO cohort were similar to that seen in our control cohort and to that seen in previously published sequencing studies of MDS as well as our control cohort, including restricting the control cohort to males only and those with a similar age spectrum to the AO exposed population,” the authors pointed out. “Thus, the spectrum of driver mutations seen in MDS exposed to AO is similar to that seen in de novo MDS.”
Noting that MDS is primarily a disease of the elderly with median age at diagnosis between 71-76, researchers advised that most of the mutations they identified were “consistent with the aging related mutational signature that has been previously reported in MDS and acute myeloid leukemia.”
“In summary, here we report the first detailed genetic characterization of MDS in patients exposed to AO during the Vietnam War era. The landscape of driver mutations and mutational events detected in this population is similar to that seen in de novo MDS and suggests that if AO promotes the development of MDS, it does so through a mechanism that is not associated with increased DNA damage,” the authors concluded.
They cautioned, however, “While we did not identify an association between any recurrently mutated genes or a clear mutational signature and AO exposure, the limited size of this study does not exclude the possibility of an AO-induced carcinogenic process that promotes the development of MDS.”
- Shallis RM, Gore SD. Agent Orange and dioxin-induced myeloid leukemia: a weaponized vehicle of leukemogenesis. Leuk Lymphoma. 2022 Feb 2:1-10. doi: 10.1080/10428194.2022.2034156. Epub ahead of print. PMID: 35105250.
- Khawandanah MO, Chaudry S, Morton J, Vidal G, et. al. Survival of acute myelogenous leukemia patients who served in the military compared to civilians. Journal of Clinical Oncology 2016 34:15_suppl, e18524-e18524.
- Sperling AS, Leventhal M, Gibson CJ, Ebert BL, Steensma DP. Myelodysplastic syndromes (MDS) occurring in Agent Orange exposed individuals carry a mutational spectrum similar to that of de novo MDS. Leuk Lymphoma. 2020 Mar;61(3):728-731. doi: 10.1080/10428194.2019.1689394. Epub 2019 Nov 12. PMID: 31714164; PMCID: PMC7268906.
- Menssen AJ, Walter MJ. Genetics of progression from MDS to secondary leukemia. Blood. 2020 Jul 2;136(1):50-60. doi: 10.1182/blood.2019000942. PMID: 32430504; PMCID: PMC7332895.
