No medications have been Food and Drug Administration-approved to treat Long COVID, which can involve post-acute symptoms in multiple organs after infection with SARS-CoV-2. A recent study suggested, however, that Paxlovid, which is prescribed to patients infected with SARS-CoV-2, who are at risk for severe symptoms, also might help protect against the condition. Researchers found the antiviral also lowered the likelihood of post-acute death and hospitalization.
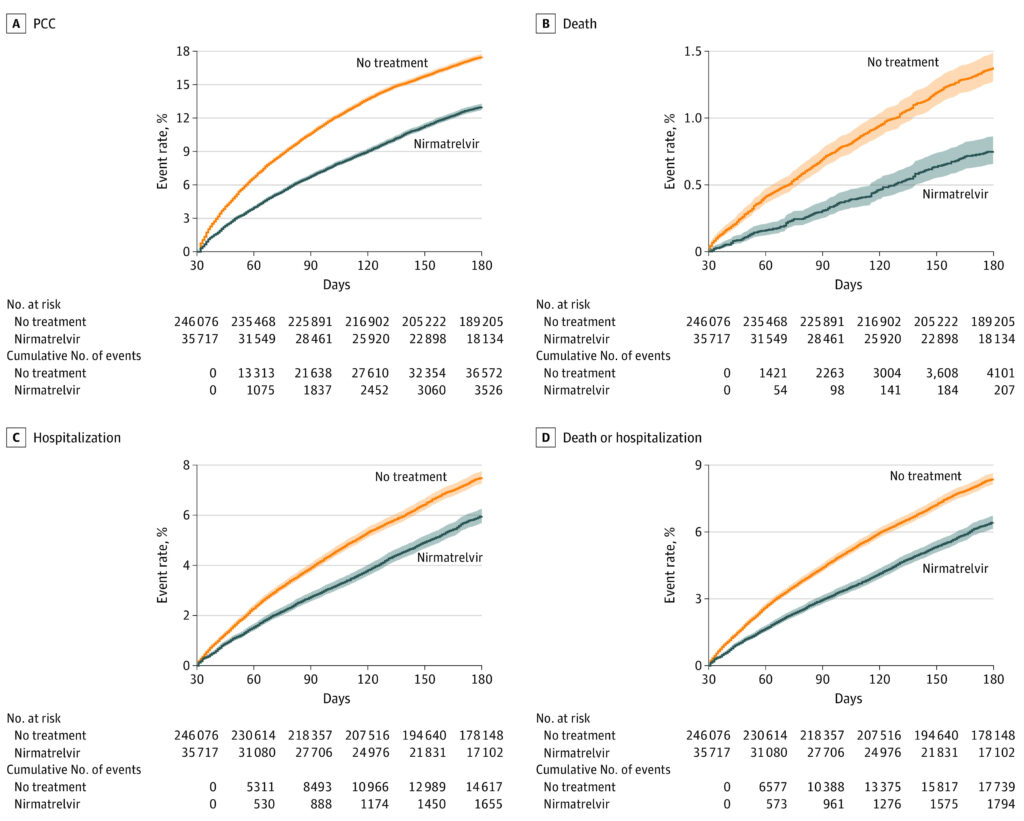
Click to Enlarge: Event Rates of Post–Acute Outcomes in Nirmatrelvir and No-Treatment Control Group
A, Post–COVID-19 condition (PCC). B, Death. C, Hospitalization. D, Composite outcome of death or hospitalization. Outcomes were ascertained 30 days after the SARS-CoV-2 positive test until the end of follow-up. Event rates in percentage presented for the nirmatrelvir group (blue, n = 35 717) and the control group (orange, n = 246 076). Shaded areas are 95% CIs. Source: JAMA Internal Medicine
ST.LOUIS — The antiviral nirmatrelvir which, in combination with ritonavir, is marketed as Paxlovid, is known to reduce the risk of progression to severe acute COVID-19. But does the oral protease inhibitor also make it less likely that patients will suffer a post–COVID-19 condition (PCC)?
That was the question addressed by the St. Louis Veterans Affairs (VA) Healthcare System and the Washington University School of Medicine. Researchers conducted a cohort study of 281,793 patients who had at least one risk factor for progression to severe COVID-19 illness.
The study, published in JAMA Internal Medicine, found that, compared with 246,076 patients who had no treatment, nirmatrelvir use in the acute phase was associated with reduced risk of PCC.1
The researchers determined reduced risk of 10 of 13 post-acute sequelae in a range of organ systems, as well as reduced risk of post-acute death and post-acute hospitalization. In addition, the authors note that nirmatrelvir was associated with reduced risk of PCC in patients who were unvaccinated, vaccinated, and boosted, as well as those with primary SARS-CoV-2 infection and reinfection.
“In people with SARS-CoV-2 infection and at least 1 risk factor for progression to severe COVID-19 illness, treatment with nirmatrelvir during the acute phase of COVID-19 was associated with reduced risk of PCC,” they concluded.
The study pointed out that prevention of PCC, also known as long COVID, affects many COVID-19 survivors and is an urgent public health priority.
The cohort study used the health care databases of the VA to identify patients who had a SARS-CoV-2 positive test result between Jan. 3, 2022, and Dec. 31, 2022, who were not hospitalized on the day of the positive test result, who had at least one risk factor for progression to severe COVID-19 illness, and who had survived the first 30 days after SARS-CoV-2 diagnosis.
The goal was to identify those who were treated with oral nirmatrelvir within 5 days after the positive test, which numbered 35,717 and those who received no COVID-19 antiviral or antibody treatment during the acute phase of SARS-CoV-2 infection, which was the control group of 246 076.
The study team reviewed prescription records to determine treatment with nirmatrelvir or receipt of no COVID-19 antiviral or antibody treatment.
Specifically, compared with the control group, nirmatrelvir was associated with reduced risk of PCC (RR, 0.74; 95% CI, 0.72-0.77; ARR, 4.51%; 95% CI, 4.01-4.99), including reduced risk of 10 of 13post–acute sequelae (components of PCC).
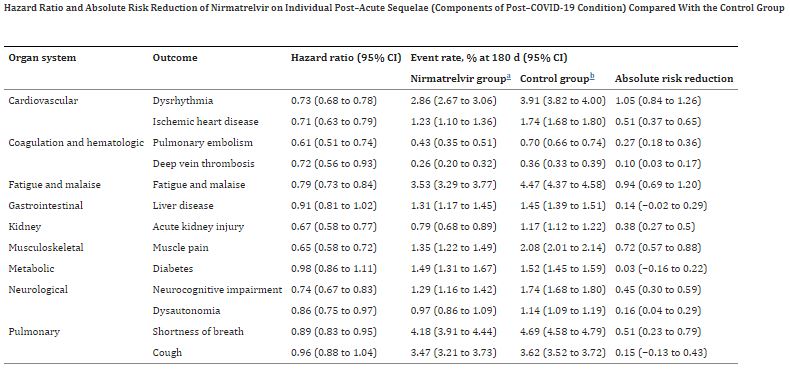
Click to Enlarge: a) The nirmatrelvir group included those who received a prescription of nirmatrelvir within 5 days after testing positive for SARS-CoV-2 and did not use any other outpatient antiviral or antibody during the first 30 days after a positive test. Outcomes were ascertained 30 days after the SARS-CoV-2 positive test until the end of follow-up.
b) The control group included those who did not use any outpatient antiviral or antibody during the first 30 days after testing positive for SARS-CoV-2 and served as the reference group in the analyses. Outcomes were ascertained 30 days after the SARS-CoV-2 positive test until the end of follow-up. Source: JAMA Internal Medicine
Those include:
- in the cardiovascular system (dysrhythmia and ischemic heart disease),
- coagulation and hematologic disorders (pulmonary embolism and deep vein thrombosis),
- fatigue and malaise,
- acute kidney disease,
- muscle pain,
- neurologic system (neurocognitive impairment and dysautonomia), and
- shortness of breath.
Nirmatrelvir was also associated with reduced risk of post-acute death (HR, 0.53; 95% CI, 0.46-0.61); ARR, 0.65%; 95% CI, 0.54-0.77), and post-acute hospitalization (HR)
The authors stated that “the totality of findings suggests that treatment with nirmatrelvir during the acute phase of COVID-19 may reduce the risk of post-acute adverse health outcomes.”
They added, “These results show that the salutary effect of nirmatrelvir may extend to the post-acute phase of COVID-19; nirmatrelvir was associated with reduced risk of PCC in the overall cohort and in various subgroups, including those across risk strata, vaccination status, and prior history of COVID-19. These findings are coupled with the observation that among 281,793 people with acute SARS-CoV-2 infection who had at least 1 risk factor for progression to severe disease who would be eligible for treatment with nirmatrelvir, 35 717 (12.67%) patients were treated with nirmatrelvir, and 246 076 (87.33%) patients received no antiviral treatment. The totality of evidence suggests that improving the uptake and use of nirmatrelvir in the acute phase as a means of not only preventing progression to severe acute disease, but also reducing the risk of post-acute adverse health outcomes may be beneficial.”
The study noted that nirmatrelvir was associated with 26% less risk of PCC, 47% less risk of post-acute death and 24% less risk of post-acute hospitalization, adding, “the magnitude of risk reduction on the absolute scale is also substantial amounting to 4.51, 0.65, and 1.72 less cases of PCC, post-acute death and post-acute hospitalization for every 100 treated persons between 30 to 180 days of infection.”
The researchers advised that their findings “should be contextualized within the broader body of evidence showing effectiveness of nirmatrelvir in also reducing risk of hospitalization or death in the acute phase. The clinical decision to initiate treatment with nirmatrelvir should consider its overall effectiveness in reducing burden of death and disease in both the acute and post-acute phases of COVID-19.”
In July, the U.S. Food and Drug Administration revised the Emergency Use Authorization (EUA) for Paxlovid to authorize state-licensed pharmacists to prescribe the drug to eligible patients, with certain limitations to ensure appropriate patient assessment and prescribing.
“The FDA recognizes the important role pharmacists have played and continue to play in combatting this pandemic,” Patrizia Cavazzoni, MD, director for the FDA’s Center for Drug Evaluation and Research, said at the time. “Since Paxlovid must be taken within five days after symptoms begin, authorizing state-licensed pharmacists to prescribe Paxlovid could expand access to timely treatment for some patients who are eligible to receive this drug for the treatment of COVID-19.”
- Xie Y, Choi T, Al-Aly Z. Association of Treatment With Nirmatrelvir and the Risk of Post–COVID-19 Condition. JAMA Intern Med. Published online, March 23, 2023. doi:10.1001/jamainternmed.2023.0743
